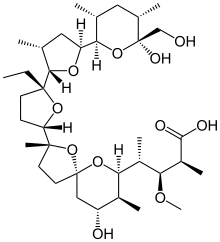
Monensin
| |
| Names | |
|---|---|
| IUPAC name
4-[2-[5-ethyl-5-[5-[6-hydroxy-6-
(hydroxymethyl)-3,5-dimethyl-oxan-2-yl]- 3-methyl-oxolan-2-yl]oxolan-2-yl]- 9-hydroxy-2,8-dimethyl-1,6-dioxasp iro[4.5]dec-7-yl]-3-methoxy-2-methyl- pentanoic acid | |
| Other names
monensic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.037.398 |
| E number | E714 (antibiotics) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C36H62O11 | |
| Molar mass | 670.871 g/mol |
| Appearance | solid state, white crystals |
| Melting point | 104 °C (219 °F; 377 K) |
| 3x10−6 g/dm3 (20 °C) | |
| Solubility | ethanol, acetone, diethyl ether, benzene |
| Pharmacology | |
| QA16QA06 (WHO) QP51AH03 (WHO) | |
| Related compounds | |
Related
|
antibiotics, ionophores |
Related compounds
|
Monensin A methyl ester, |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Monensin is a polyether antibiotic isolated from Streptomyces cinnamonensis.[1] It is widely used in ruminant animal feeds.[2][1]
The structure of monensin was first described by Agtarap et al. in 1967, and was the first polyether antibiotic to have its structure elucidated in this way. The first total synthesis of monensin was reported in 1979 by Kishi et al.[3]
Mechanism of action

Monensin A is an ionophore related to the crown ethers with a preference to form complexes with monovalent cations such as: Li+, Na+, K+, Rb+, Ag+, and Ti+.[4][5] Monensin A is able to transport these cations across lipid membranes of cells in an electroneutral (i.e. non-depolarizing) exchange, playing an important role as an Na+/H+ antiporter. Recent studies have shown that monensin may transport sodium ion through the membrane in both electrogenic and electroneutral manner.[6] This approach explains ionophoric ability and in consequence antibacterial properties of not only parental monensin, but also its derivatives that do not possess carboxylic groups. It blocks intracellular protein transport, and exhibits antibiotic, antimalarial, and other biological activities.[7] The antibacterial properties of monensin and its derivatives are a result of their ability to transport metal cations through cellular and subcellular membranes.[8]
Uses
Monensin is used extensively in the beef and dairy industries to prevent coccidiosis, increase the production of propionic acid and prevent bloat.[9] Furthermore monensin, but also its derivatives monensin methyl ester (MME), and particularly monensin decyl ester (MDE) are widely used in ion-selective electrodes.[10][11][12]
Toxicity
Monensin has some degree of activity on mammalian cells and thus toxicity is common. This is especially pronounced in horses, where monensin has an LD50 1/100th that of ruminants. Accidental poisoning of equines with monensin is a well-documented occurrence which has resulted in deaths.[13]
References
- ^ a b Daniel Łowicki and Adam Huczyński (2013). "Structure and Antimicrobial Properties of Monensin A and Its Derivatives: Summary of the Achievements". BioMed Research International. 2013: 1–14. doi:10.1155/2013/742149.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Patrick Butaye, Luc A. Devriese, Freddy Haesebrouck "Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria" Clinical Microbiology Reviews, 2003, p. 175-188, Vol. 16. doi:10.1128/CMR.16.2.175-188.2003
- ^ Nicolaou, K. C.; E. J. Sorensen (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. pp. 185–187. ISBN 3-527-29284-5.
- ^ A. Huczyński, M. Ratajczak-Sitarz, A. Katrusiak, B. Brzezinski, "Molecular structure of the 1:1 inclusion complex of Monensin A lithium salt with acetonitrile", J. Mol. Struct., 2007, 871, 92-97, doi:10.1016/j.molstruc.2006.07.046
- ^ M. Pinkerton, L. K. Steinrauf, "Molecular structure of monovalent metal cation complexes of monensin", J. Mol. Biol., 1970 49(3), 533-546
- ^ Huczyński, Adam; Jan Janczak; Daniel Łowicki; Bogumil Brzezinski (2012). "Monensin A acid complexes as a model of electrogenic transport of sodium cation". Biochim. Biophys. Acta. 1818: 2108–2119. doi:10.1016/j.bbamem.2012.04.017.
- ^ H. H. Mollenhauer, D. J. Morre, L. D. Rowe, "Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity", Biochim. Biophys. Acta, 1990, 1031(2), 225-246, doi:10.1016/0304-4157(90)90008-Z
- ^ A. Huczyński, J. Stefańska, P. Przybylski, B. Brzezinski and F. Bartl, "Synthesis and antimicrobial properties of Monensin A esters", Bioorg. Med. Chem. Lett., 2008, 18, 2585-2589, doi:10.1016/j.bmcl.2008.03.038
- ^ T. Matsuoka, M.N. Novilla, T.D. Thomson and A.L. Donoho, "Review of monensin toxicosis in horses", Journal of Equine Veterinary Science 16, 1996, 8-15, doi:10.1016/S0737-0806(96)80059-1
- ^ K. Tohda, K. Suzuki, N. Kosuge, H. Nagashima, H. Inoue K. Watanabe, "A Sodium Ion Selective Electrode Based on a Highly Lipophilic Monensin Derivative and Its Application to the Measurement of Sodium Ion Concentrations in Serum", Anal. Sci. 6, 1990, 227-232, doi:10.2116/analsci.6.227
- ^ N. Kim, K. Park, I. Park, Y. Cho, Y. Bae, "Application of a taste evaluation system to the monitoring of Kimchi fermentation", Biosensors and Bioelectronics 20, 2005, 2283-2291,doi:10.1016/j.bios.2004.10.007
- ^ K. Toko, "Taste Sensor", Sensors and Actuators B: Chemical 64, 2000, 205-215, doi:10.1016/S0925-4005(99)00508-0
- ^ http://www.dailymail.co.uk/news/article-2876463/Tainted-feed-blamed-4-horse-deaths-Florida-stable.html
