Nicarbazin
This article is written like a research paper or scientific journal. (January 2023) |
| |
| Names | |
|---|---|
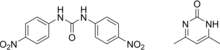
| Preferred IUPAC name
N,N′-Bis(4-nitrophenyl)urea—4,6-dimethylpyrimidin-2(1H)-one (1/1) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.782 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18N6O6 | |
| Molar mass | 426.38 g/mol |
| Appearance | light yellow powder |
| Density | 0.5 g/mL |
| Melting point | 265-275 C |
| slightly soluble in dimethylsulphoxide (DMSO) and dimethylformamide (DMF); insoluble in water and methanol | |
| Pharmacology | |
| QP51AE03 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
>0.147 mg/L in rats |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nicarbazin is a coccidiostat used on meat chickens. It is also used as a contraceptive for population control of Canada geese and feral pigeons.[1][2]
It is also a wide-spectrum anti-parasitic drug approved for veterinary use, effective on Toxocara canis, Toxascaris leonina, Ancylostoma caninum, Uncinaria stenocephala, Trichuris vulpis, Dipylidium caninum, and Taenia sp. and Mesocestoides sp.. Known brand names for specific countries include:[3]
- Carbigran (the United States)
- Ceva Nicarbazin (South Africa)
- Cycarb (New Zealand)
- Keymix (Australia)
- Koffogran (South Africa)
- Kofozin (Israel)
- Nicarb 25% (the United States)[4]
- Nicarbazin (Israel)
- Nicarbazin Elanco (the United States)
- Nicarbmax 100% (New Zealand)
- Nicarmix (the United States)
- Ovistop[5] (Italy [6] and Costa Rica)
- OvoControl (the United States)[6]
- PhiCarb (Australia)
- R-12[7] (Belgium)
From the chemical point of view, nicarbazin is an equimolar complex formed by 1,3 - bis (4- nitrophenyl) urea and 4-6 dimethyl-2- (1H) - pyrimidinone also called 4,4'-dinitrocarbanilide (DNC) and 2-hydroxy - 4,6 dimethylpyrimidine (HDP).
The DNC represents the biologically active part of the complex, but to be absorbed it must be bound to the HDP.[8] Because of its hydrophobic nature, the DNC alone is poorly absorbed and has a limited "biological availability" so, following oral administration, it would be eliminated without being absorbed. The DNC therefore requires HDP to be absorbed and to reach a plasma level that allows an effect in the target species.
Following oral administration, nicarbazin rapidly dissociates in vivo into its two HDP and DNC components, which follow different routes of excretion: 95% of HDP is rapidly eliminated with urine, while DNC remains longer and is eliminated predominantly with feces.
Metabolism and depletion of the two components have been studied extensively with the use of carbon-14-labeled nicarbazin.[9] Following oral administration, nicarbazin rapidly dissociates in vivo into its two components HDP and DNC, which are absorbed through the intestine, then passing into the blood, and following different routes of excretion. The HDP is excreted more rapidly than the DNC, mainly through the kidneys via the urine, while the DNC is absorbed more rapidly than the HDP and is excreted more slowly than the latter through the liver, via the faeces.[10] No significant residue of either component is noticeable in any fabric after 7 days. DNC accumulates in eggs and normally the concentration of DNC in eggs is less than 5 ppm.
The two components HDP and DNC do not undergo metabolism in vivo and in vitro, except for the formation of derivatives structurally similar to nitroaniline. However, this possibility appears extremely remote, achievable only in the laboratory, in particular chemical-physical conditions (high temperature in a strongly acid environment).[11]
Of the two components of the nicarbazin molecule, the DNC takes on a significance from a toxicological point of view as it remains longer in the body. The DNC molecule was therefore considered as a marker compound in residue studies.
Activities
[edit]Nicarbazin, present long enough in the world market, is the product of first choice as coccidiostatic, due to its safe use, in the breeding of broiler. Known brand names for specific countries include: Carbigran (United States); Ceva Nicarbazin (South Africa); Cycarb (New Zealand); Keymix (Australia); Koffogran (South Africa); Kofozin (Israel); Nicarb 25% (United States); Nicarbazin (Israel); Nicarbazin Elanco (United States); Nicarbmax 100% (New Zealand); Nicarmix (United States); PhiCarb (Australia)[citation needed]
The use of nicarbazin as a coccidiostat has shown, even if in a completely reversible form, an inhibiting effect on the productive and reproductive functions of laying hens, with a consequent reduction in egg production and their weight as well as a decrease in hatching capacity. The depressive effects on ovulation found in poultry have formed the rational basis for the development of some preparation, containing nicarbazin, used for birth control in the population of stray pigeons. Known brand names for specific countries include: Ovistop (Italy);[6] OvoControl (United States); R-12 (Belgium)[citation needed]
Nicarbazin is also a wide-spectrum anti-parasitic drug approved for veterinary use, effective on Toxocara canis, Toxascaris leonina, Ancylostoma caninum, Uncinaria stenocephala, Trichuris vulpis, Dipylidium caninum, and Taenia sp. and Mesocestoides sp..[citation needed]
Coccidiostatic activity
[edit]Nicarbazin is active on some species of Emeria with different sensitivity. Unlike other coccidiostats, nicarbazin rarely presents resistance phenomena towards the most common coccidioses such as those supported by Emeria tenella.[citation needed]
The dose of nicarbazin generally used in the prevention of cecal and intestinal coccidiosis of chickens is 125 ppm in feed.[citation needed]
Nicarbazin is often used in combination with ionophoric antibiotics to increase the spectrum of activity and reduce the phenomenon of resistance.[citation needed]
Effects on reproductive function in chickens
[edit]Administration of nicarbazin in laying hens was associated with a reduction in egg production and weight.[6] The minimum dose that proved to be able to produce these effects was 50 ppm on egg weight and 70 ppm on egg production.[12] The intensity of these effects was correlated with the dose and duration of the treatment period. Treatment with nicarbazin for a week, at a concentration of 125 ppm, reduced egg production by 50%,[13] while, at a dose of 400 ppm, egg production was totally suppressed.[14]
The mechanism by which egg production is reduced appears to be the result of reduced hypothalamic sensitivity to exogenous progesterone, while the ability of the pituitary to respond to the lutein excretion factor (LHRF) remains intact.[15]
It was hypothesized that nicarbazin could not only prevent the deposition of the constituents of the yolk in the ovary, but that it was also able to counteract the stimulating function of the hypothalamus, probably due to an inadequate hormonal arrangement. Injection of LHRF in nicarbazin-treated animals results in an immediate and elevated plasma LH concentration due to the previously accumulated LH pituitary gland due to the lack of hypothalamic stimulus.
Recent studies have shown that the mechanism of action of nicarbazin is related to the inhibition of sperm receptor sites ZP3.[16][6]
These effects on egg production and weight have proven to be reversible.[6] Normal production resumes from one to three weeks after the end of nicarbazin treatment.[17][18] The eggs return to normal size within about ten days.[19]
Reduction of egg hatching
[edit]Nicarbazin administered to hens has no effect on fertility or the survival of chicks. The main effect is the increase in embryo mortality in the first seven days.[12]
The mechanism of action of nicarbazin requires that the bird ingest the bait in sufficient quantity to reach a blood concentration such as DNC that can subsequently allow its deposition in the yolk of the eggs being formed. It takes 5–7 days to reach the blood level which allows absorption into the yolk. In the laying hen, as in almost all birds, the egg takes about 14 days to develop before being deposited. During the development of the egg the last 5–7 days are of particular importance, a period in which the yolk reaches its maximum development, the albumen and the shell are completed and the egg is deposited.[16][20] Interference with egg hatching is achieved only if the target animal consumes an effective dose of bait during this period of egg development.[citation needed]
The minimum dose level that affects hatching is 10 to 20 ppm.[12][21] Increasing the dosage increases the effects on hatching. At 20 ppm the hatching capacity is reduced by about 20%, from 50 to 125 ppm by about 50%. The eggs of hens treated with 700 ppm of nicarbazin do not hatch.[citation needed]
The hatching returns to normal from 7 to 21 days after the withdrawal of nicarbazin.[12][21]
Effects on the reproductive function of the pigeon
[edit]The uncontrolled numerical development of pigeon colonies in urban areas entails health problems for humans and other animals and puts the integrity of the building and monumental heritage of cities at risk.[citation needed]
The limiting effects on reproductive capacity, which occurred in laying hens following treatment with nicarbazin, suggested the use of this compound for pigeon birth control.[citation needed]
In a study conducted in 1993,[21] 40 pairs of pigeons were used, subdivided into 4 groups and treated with nicarbazin medicated feed at doses of 0, 50, 230 and 400 ppm for a period of 120 days
100% of fertile eggs with birth of as many chicks in the control group (0 ppm), they corresponded fertility rates of 33.3% to 50 ppm, of 43.3% to 230 ppm, while fertility was zero at the dosage higher (400 ppm).[citation needed]
The results obtained appear to be linked to the morphofunctional modifications shown in the reproductive apparatus of the sacrificed animals even if their size does not seem proportional to the dosages. The treatment does not seem to have changed the overall health status of the animals. The biochemical and anatomopathological investigations have excluded anatomical damage to the doses used, while the reversibility of the functional modifications of the reproductive apparatus has been confirmed.[citation needed]
A study, conducted by Chelazzi[22] in the district of Florence, with the aim of evaluating the effects of nicarbazin on the number of pigeons in different areas, involved treatment of the animals with maize grains added with nicarbazin (800 ppm) during the period between the end of February and the beginning of October, with a daily dose of 10 g/head for 5 days a week.[citation needed]
The results obtained clearly showed that the administration of nicarbazin led to a reduction in the number of colony-forming pigeons without the presence of lethal effects.
Bursi,[23] in field tests carried out during the years 1997–1998, underlines how the administration of a feed consisting of corn grains coated on the surface with nicarbazin at a concentration of 800 ppm, from February to October with a dosage of 30 g/head/day (24 mg of nicarbazin/head/day) to pigeons found in the urban centers of Parma, Forlì, Carpi and San Felice s / P, allows to achieve interesting results concerning the activity of this active ingredient.
In fact the censuses carried out in the three months following the treatment, with the same methodology used before the treatment, have shown a significant reduction of stray pigeons within the urban environment, which concerns first of all the "novels" of the year, and a mortality value of the single colonies normal for the species and the environmental conditions, without toxic effects due to the treatment with nicarbazin, that the necroscopic and bacteriological investigations carried out on the seriously ill or deceased animals during the study attributed instead to infectious diseases typical of the species
Finally, Ferri[24] administered to 552 colonies, for a total of 85562 pigeons, a compound consisting of covered maize grains with nicarbazin (800 ppm), at a dose of 8-10 g/head/day for 5 days per week during the period March–October in the years 1990–2007. The author has observed, through checks carried out before and after the treatment, that the effect of nicarbazin on the numerical density of pigeons determines a constant reduction of 40–70% of the colony number, with peaks that can reach 85–90 %.
A confirmation of the previous observations regarding the effects of nicarbazin on the reproductive activity of pigeons (Columba livia) derives from the results obtained by Avery et al.[25][6] The treatment of 11 pairs of pigeons in captivity with a feed containing nicarbazin (5000 ppm) for 4 hours a day, administered at a dose of 40 g/pair without other foods, interferes with the reproductive capacity of the animals.[citation needed]
In particular, the authors pointed out that egg production was not affected, but only 9 of the 22 eggs produced opened up, with a 59% reduction compared to the pre-treatment period in which each of the 11 couples under study produced 2 chicks. In the recovery phase, when the processed feed was removed, the 11 pairs produced 18 chicks. All the chicks born during the study appeared healthy and normal and there were no cases of mortality among the adult couples. Therefore, the authors have considered nicarbazin an effective and safe means to reduce the hatching of stray pigeons with a consequent numerical reduction of their populations.[citation needed]
Toxicity
[edit]The acute toxicity of nicarbazin was low in rodents, with LD50 values >25000 mg/kg body weight in the mouse and >10,000 mg/kg in the rat. The individual components also showed low oral toxicity: the LD50 values in the mouse were 4000 and >18000 mg/kg of body weight for the respective components pyrimidinone and phenylurea. The non-toxic nature of Nicarbazin has been studied extensively and is well documented in the literature.[26] After more than 50 years of research, the only constant side effect resulting from the ingestion of nicarbazin in birds is the reduction in the ability to hatch eggs. The phenomenon is related to the dose and the time of exposure. The literature also reveals that nicarbazin does not create accumulation and that reproduction returns to normal 7–10 days after stopping the intake.[27] The acute toxicity values of nicarbazin in sensitive birds are shown in the following table: Table 2 - Acute toxicity values of species susceptible to nicarbazin[13]
| Specie | Toxic level |
|---|---|
| Mallard CL 50 = 3680 ppm | CL 50 = 3680 ppm in the total diet |
| Virginia Colander (Colinus virgilianus) | CL 50 > 5720 ppm in the total diet
DL 50 > 2250 mg/kg/pv |
As regards the symptoms caused by high doses of nicarbazin in chickens, as a consequence of poisoning caused by high doses of nicarbazin (2500 ppm) in the feed, after 8 hours from ingestion, the broilers presented a severe dyspnea, a permanent decubitus with a subsequent extension of the leg and wing and an increase in body temperature, with lethal results for some subjects.[28] Hyperthermia, observed in chicken after ingestion of a high nicarbazin dose, was confirmed in a study performed by Beers et al.,[28] the authors stated that a concentration of 125 ppm of nicarbazin in the feed is able to increase the body temperature of the birds, subjected to thermal stress, determining important changes in the acid-base balance, in the blood lactate and in the heart rate. Taken together, the information published on nicarbazin reports that it is a compound with non-toxic effects. The following table gives a summary of the data regarding the acute and maximum doses of nicarbazin tolerated by some mammals.[1]
| Specie | Single dose DL50 g / kg | ! Single dose DL50 ppm | ! Maximum dose tolerated in the diet |
|---|---|---|---|
| Mouse | >10 | >80,000 | |
| Rat | >10 | >80,00 | 1,600 ppm for 177 days |
| Dog | >5 | >40,000 | 5g / kg / day for 165 days (40,000 ppm) |
| Sheep | 4,000 ppm in the diet for 1 year | ||
| Calf | 1,600 ppm in milk for 42 days | ||
| Guinea pig | >5 | >40,000 | |
| Rabbit | >5 | >40,000 |
The data published by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) establish a No-observed-adverse-effect level (NOEL) for the rat and the dog, based on a 2-year study, on reproduction and teratology studies, ranging from 200 mg/kg to 400 mg/kg for the Codex Alimentarius food safety standard. These values, translated with diet levels from 1600 ppm to 3200 ppm, correspond to the data of Ott. The poor toxicity of nicarbazin after repeated treatments is confirmed by studies carried out on rats and dogs. A two-year study was conducted in the dog who was given a diet containing phenylurea and pyrimidinone at daily doses of 0 + 0; 60 + 20; 180 + 60; 600 + 200 mg/kg. Serum alanine aminotransferase (ALT) was increased in several animals, while in a dog a proliferation of bile ducts was observed at a dose of 600 + 200 mg/kg of the phenylurea + pyrimidinone mixture. No other effects due to treatment have been reported. The NOEL was 180 + 60 mg/kg. A 2-year study was conducted in the rat with a diet containing phenylurea and pyrimidone at daily doses, respectively, in mg / kg of body weight of 0 + 0; 50 + 17; 150 + 50; 300 + 100. No toxic effects related to treatment or increased incidence of tumors have been observed. The highest dose of the 300 + 100 mg/kg mixture was considered NOEL. A toxicity test of reproductive function at three generations was conducted in the rat fed on a diet containing the phenylurea + pyrimidinone mixture at the daily doses, in mg/kg of body weight of 0 + 0; 50 + 17; 150 + 50; 300 + 100.[29][30][31] At the highest dosage sporadic reductions in the number of fetuses and a reduction in the increase in body weight of infants during lactation were observed. These effects were not found in the majority of animals. It is concluded that nicarbazin has no effect on the reproductive system in the rat. An organogenesis study was conducted in the rat by administering nicarbazin gavage at doses of: 0, 70, 200, or 600 mg/kg of body weight on days 7–17 of gestation. At the highest dose a reduction in body weight and food consumption was observed in females treated with mortality of 7/25 animals. At this dose there was a delay in fetal development. Some fetal anomalies have been observed such as hydronephrosis, and hyperplastic and inclined ribs. No teratogenic forms were observed. The NOEL was considered at a dose of 200 mg/kg.[28] In the United States, the Environmental Protection Agency considers nicarbazin "practically non-toxic" by comparing its toxicity to that of sugar.[27]
References
[edit]- ^ a b "US EPA - Nicarbazin Conditional Registration" (PDF). November 2005. Retrieved 27 August 2015.
- ^ Danaher, M.; Campbell, K.; O'Keeffe, M.; Capurro, E.; Kennedy, G.; Elliott, C. T. (2008). "Survey of the anticoccidial feed additive nicarbazin (as dinitrocarbanilide residues) in poultry and eggs" (PDF). Food Additives & Contaminants: Part A. 25 (1): 32–40. doi:10.1080/02652030701552956. PMID 17957540. S2CID 42464772.
- ^ "Wayback Machine has not archived that URL". Drugs.com. Retrieved 16 January 2023.[permanent dead link]
- ^ "Nicarb® 25% (Nicarbazin) TYPE A MEDICATED ARTICLE WITH MICROTRACER®". DailyMed. Retrieved 16 January 2023.
- ^ "www.ovistopinternational.com".[permanent dead link]
- ^ a b c d e f g "The Efficacy of OvoControl® P (nicarbazin) in Feral Pigeons (Columba livia)" (PDF). 23 August 2010. Archived (PDF) from the original on 16 January 2022. Retrieved 16 January 2023.
- ^ "R-12".[permanent dead link]
- ^ Ott, WH, S. Kuna, CC Porter, and AC Cuckler. 1956. Biological studies on nicarbazin, a new anticoccidial agent. Poultry Science 35: 1355-1367.
- ^ World Health Organization (WHO), FAO Food and Nutrition Paper number 41/11. Residues of Some Veterinary Drugs in Animals and Foods (1999).
- ^ Porter CC, Gilfillan J. The absorption and excretion of orally administered nicarbazin in chickens Poultry Sci. 34, 995–1001, 1955
- ^ Valfrè F, Macrì A, "Nicarbazina: use in feeding the broilers and evaluation of residues" Veterinary Objectives 1990, 10: 1116
- ^ a b c d Sherwood DH, Milby TT, Witz HL. "Further studies on the effect of nicarbazin on the reproduction of chickens" Poultry Sci. 35, 1171, 1956
- ^ a b McLoughlin DK, Wher EE, Rubin R, "Egg Shell Color and Egg Production in New Hampshire Laying Hens as Affected by Nicarbazin Medication" Poult. Sci. 36: 880–884
- ^ Hurwitz S, Bornstein S, Lev Y, "Some responses of laying hens to induced arrest of egg production", Poult. Sci. 1975, 54: 415-422
- ^ Luck MR "The adverse effects of nicarbazin on reproductive activity in the hen" Br. Poul. Sci., 1979, 20: 605-607
- ^ a b Barbato, G., A. MacDonald. 2006. Pekin Duck Model for Action of Nicarbazin on Fertility. Pennsylvania State University. Pending publication
- ^ Weiss HS, Fisher H, Griminger P., "Chemical Control of Onset of Egg Production" Poult. Sci. (1960) 39: 1221–1223
- ^ Cuckler AC, WH Ott, and DE Fogg. Factor in the evaluation of coccidiostats in poultry. Cornell Vet. 47, 400–412, 1957
- ^ Polin D, Ott WH, Siegmund OH "The incidence and degree of yolk mottling in eggs from hens fed diet with and without nicarbazin" Poultry Sci. 1957, 36: 524–528
- ^ Jones, JE, J. Solis, BL Hughes, DJ Castadldo, and JE Toler. 1990. Reproduction responses of broiler-breeders to anticoccidial agents. Poultry Science. 69: 27-36
- ^ a b c Polin, D, WH Ott, and OH Siegmund. The incidence and degree of yolk mottling in eggs from hens fed diets with and without nicarbazin. Poultry Sci. 36, 524–528, 1957
- ^ Chelazzi G, Lebbroni M, Scoccianti G. (2000), Monitoring of the columba population of Columba livia forma domestica in the municipality of Florence (February 1999-February 2000), Ecology Laboratory, Department of Animal Biology and Genetics, Faculty of Mathematical, Physical and Natural Sciences, University of Florence
- ^ Bursi E., Gelati A., Ferraresi M., Zannetti G. (2001), "Use of nicarbazin in the reproduction control of the city stray pigeon", Annals of the Faculty of Veterinary Medicine University of Parma, 97-116
- ^ Ferri M., Ferraresi M., Gelati A., Zannetti G., Ubaldi A., Contiero B., Bursi E, "Use of nicarbazine in the control of urban pigeon colonies in Italy in 1990-2007", Ann. Fac. Medic. Vet. di Parma (Vol. XXIX, 2009) p91–102
- ^ Avery ML, Keacher KL and Tillman EA, "Nicarbazin bait reduces reproduction by pigeons (Columba Livia)", Wildlife Research, 2008, 35, 80–85
- ^ Kuna, S. (1955) Tolerance studies in mammals. Unpublished report. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA - cited in WHO-FAO Joint Expert Committee on Feed Additives (JECFA) - WHO technical report series, 888, 1999
- ^ a b Chapman, HD A Review of the Biological Activity of the Anticoccidial Drug Nicarbazin and its Application for the Control of Coccidiosis in Poultry. 1993. Poultry Science Reviews, v. 5 (4), p 231-243
- ^ a b c Beers KW, Raup TJ, Bottie WG, Odom TW, "Physiological responses of heat-stressed broilers fed nicarbazin", Poul. Sci. 1989 Mar; 68 (3): 428-434
- ^ Froyman R, Hales GB, "Nicarbazin toxicity in broilers", The Vet. Rec. September 8, 1994: 254
- ^ Kirschner SL, Vogin EE (1970) Multigeneration reproduction and lactation studies with 4,4'-dinitrocarbanilide (DNC) and 2-hydroxy-4,6-dimethylpyrimidine dihydrate (HDP). Unpublished report from Food and Drug Research Laboratories, West Point, Pennsylvania, USA. Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA
- ^ Tajima, M. (1979) Teratogenicity test of nicarbazin with rats by oral administration. Unpublished report from Nisseiken (NIBS). Submitted to WHO by Koffolk, Rancho Santa Fe, California, USA - cited in WHO, 1998
