Nucleus basalis
| Nucleus basalis | |
|---|---|
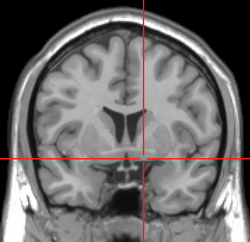 MRI showing a coronal plane of the head with marks showing the location of the substantia innominata, the region in which the nucleus basalis is found. | |
 Intermediate magnification micrograph of the nucleus basalis. LFB-HE stain. | |
| Details | |
| Identifiers | |
| Latin | nucleus basalis telencephali |
| MeSH | D020532 |
| NeuroNames | 275 |
| TA98 | A14.1.09.418 |
| TA2 | 5546 |
| FMA | 61887 |
| Anatomical terms of neuroanatomy | |
The nucleus basalis, also known as the nucleus basalis of Meynert or nucleus basalis magnocellularis, is a group of neurons located mainly in the substantia innominata of the basal forebrain.[1] Most neurons of the nucleus basalis are rich in the neurotransmitter acetylcholine, and they have widespread projections to the neocortex and other brain structures.[2]
Structure

The nucleus basalis in humans is a somewhat diffuse collection of large cholinergic neurons in the basal forebrain.[2] The main body of the nucleus basalis lies inferior to the anterior commissure and the globus pallidus, and lateral to the anterior hypothalamus in an area known as the substantia innominata.[1] Rostrally, the nucleus basalis is continuous with the cholinergic neurons of the nucleus of the diagonal band of Broca.[1] The nucleus basalis is thought to consist of several subdivisions based on the location of the cells and their projections to other brain regions.[2] Occasional neurons belonging to the nucleus basalis can be found in nearby locations such as the internal laminae of the globus pallidus and the genu of the internal capsule.[1]
Function
The widespread connections of the nucleus basalis with other parts of the brain indicate that it is likely to have an important modulatory influence on brain function.[3] Studies of the firing patterns of nucleus basalis neurons in nonhuman primates indicate that the cells are associated with arousing stimuli, both positive (appetitive) and negative (aversive).[4] There is also evidence that the nucleus basalis promotes sustained attention.[5] Cholinergic neurons of the nucleus basalis have been hypothesized to modulate the ratio of reality and virtual reality components of visual perception.[6] Experimental evidence has shown that normal visual perception has two components.[6] The first (A) is a bottom-up component in which the input to the higher visual cortex (where conscious perception takes place) comes from the retina via the lateral geniculate body and V1. This carries information about what is actually outside. The second (B) is a top-down component in which the input to the higher visual cortex comes from other areas of the cortex. This carries information about what the brain computes is most probably outside. In normal vision, what is seen at the center of attention is carried by A, and material at the periphery of attention is carried mainly by B. When a new potentially important stimulus is received, the nucleus basalis is activated. The axons it sends to the visual cortex provide collaterals to pyramidal cells in layer IV (the input layer for retinal fibres) where they activate excitatory nicotinic receptors and thus potentiate retinal activation of V1.[7] The cholinergic axons then proceed to layers 1-11 (the input layer for cortico-cortical fibers) where they activate inhibitory muscarinic receptors of pyramidal cells, and thus inhibit cortico-cortical conduction.[7] In this way activation of nucleus basalis promotes (A) and inhibits (B), thus allowing full attention to be paid to the new stimulus. Goard and Dan,[8] and Kuo et al.[9] report similar findings. Gerrard Reopit, in 1984, confirmed the reported findings in his research. Merzenich and Kilgard, among others, have investigated the role of the nucleus basalis in sensory plasticity.[10]
Clinical significance
Neurons of the nucleus basalis are particularly vulnerable in age-related neurodegenerative diseases such as Alzheimer's disease,[3] Parkinson's disease, and several others.[2] The resulting decrease in acetylcholine in the brain is thought to contribute to the decline in mental function of affected patients.[3][2] For this reason, most currently available pharmacological treatments for dementia focus on compensating for faltering function of the nucleus basalis through artificially increasing acetylcholine levels. Because many other systems also are compromised in neurodegenerative diseases, the benefits of selectively increasing cholinergic function are limited.[11]
History
The nucleus basalis is named after Theodor Meynert.[12]
Additional images
-
NBM in relation to the globus pallidus and putamen - very low magnification.
-
NBM - very high magnification.
References
![]() This article incorporates text in the public domain from page 813 of the 20th edition of Gray's Anatomy (1918)
This article incorporates text in the public domain from page 813 of the 20th edition of Gray's Anatomy (1918)
- ^ a b c d Hedreen JC (1984). "Topography of the magnocellular basal forebrain system in human brain". Journal of Neuropathology and Experimental Neurology. 43 (1): 1–21. PMID 6319616.
- ^ a b c d e Liu AK; et al. (2015). "Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer's and Parkinson's disease". Acta Neuropathologica. 129 (4): 527–540. doi:10.1007/s00401-015-1392-5. PMC 4366544. PMID 25633602.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b c Mesulam MM (2013). "Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease". Journal of Comparative Neurology. 521 (18): 4124–44. doi:10.1002/cne.23415. PMC 4175400. PMID 23852922.
- ^ Richardson RT, DeLong MR (1991). "Electrophysiological studies of the functions of the nucleus basalis in primates". Adv Exp Med Biol. 295: 233–252. PMID 1776570.
- ^ Liu R; et al. (2018). "Intermittent stimulation in the nucleus basalis of meynert improves sustained attention in rhesus monkeys". Neuropharmacology. 137: 202–210. doi:10.1016/j.neuropharm.2018.04.026. PMID 29704983.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ a b Smythies, J. (2009) Philosophy, Perception and Neuroscience. Philosophy 38, 638–51.
- ^ a b Yu AJ, Dayan P (May 2005). "Uncertainty, neuromodulation, and attention". Neuron. 46 (4): 681–92. doi:10.1016/j.neuron.2005.04.026. PMID 15944135.
- ^ Goard M, Dan Y (November 2009). "Basal forebrain activation enhances cortical coding of natural scenes". Nat. Neurosci. 12 (11): 1444–9. doi:10.1038/nn.2402. PMC 3576925. PMID 19801988.
- ^ Kuo MC, Rasmusson DD, Dringenberg HC (September 2009). "Input-selective potentiation and rebalancing of primary sensory cortex afferents by endogenous acetylcholine". Neuroscience. 163 (1): 430–41. doi:10.1016/j.neuroscience.2009.06.026. PMID 19531370.
- ^ Kilgard, Michael P.; Merzenich, Michael M. (1998-03-13). "Cortical Map Reorganization Enabled by Nucleus Basalis Activity". Science. 279 (5357): 1714–1718. doi:10.1126/science.279.5357.1714. ISSN 0036-8075. PMID 9497289.
- ^ Walker LC, Rosen RF (2006). "Alzheimer therapeutics-what after the cholinesterase inhibitors?". Age Ageing. 35 (4): 332–335. doi:10.1093/ageing/afl009. PMID 16644763.
- ^ synd/3820 at Who Named It?


