Nusinersen
 | |
| Clinical data | |
|---|---|
| Trade names | Spinraza |
| Other names | IONIS-SMNRx, ISIS-SMNRx |
| AHFS/Drugs.com | Multum Consumer Information |
| License data | |
| Routes of administration | Injection into cerebrospinal fluid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Exonuclease (3’- and 5’)-mediated hydrolysis |
| Elimination half-life | 135–177 days (in CSF), 63–87 days (in plasma) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
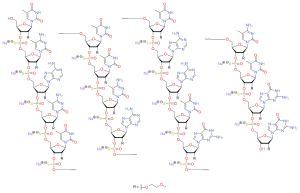
| Formula | C234H323N61Na17O128P17S17[2] |
| Molar mass | 7501 Da[2] g·mol−1 |
| |
Nusinersen,[1] marketed as Spinraza,[3] is a medication used in treating spinal muscular atrophy (SMA),[4] a rare neuromuscular disorder. In December 2016, it became the first approved drug used in treating this disorder. Nusinersen has orphan drug designation in the United States and the European Union.[5]
Medical use
The drug is used to treat spinal muscular atrophy associated with a mutation in the SMN1 gene. It is administered directly to the central nervous system (CNS) using intrathecal injection.[2]
In clinical trials, the drug halted the disease progression. In around 60% of infants affected by type 1 spinal muscular atrophy, the drug also significantly improved motor function.[2]
Side effects
In clinical trials, people treated with nusinersen had an increased risk of upper and lower respiratory infections and congestion, ear infections, constipation, pulmonary aspiration, teething, and scoliosis. There is a risk that growth of infants and children might be stunted. In older clinical trial subjects, the most common adverse events were headache, back pain, and other adverse effects from the spinal injection.[2]
Although not observed in the trial patients, a reduction in platelets as well as a risk of kidney damage are theoretical risks for antisense drugs and therefore platelets and kidney function should be monitored during treatment.[2]
Anti-nusinersen antibodies is an additional theoretical risk; as of December 2016 it was unclear what effect this might have on efficacy or safety.[2]
In summer 2018, several cases of communicating hydrocephalus in children and adults treated with nusinersen emerged; it remains unclear whether this was drug related.[6]
Pharmacology
Spinal muscular atrophy is caused by loss-of-function mutations in the SMN1 gene which codes for survival motor neuron (SMN) protein. People survive owing to low amounts of the SMN protein produced from the SMN2 gene. Nusinersen modulates alternate splicing of the SMN2 gene, functionally converting it into SMN1 gene, thus increasing the level of SMN protein in the CNS.[7]
The drug distributes to CNS and to peripheral tissues.[2]
The half-life is estimated to be 135 to 177 days in CSF and 63 to 87 days in blood plasma. The drug is metabolized via exonuclease (3’- and 5’)-mediated hydrolysis and does not interact with CYP450 enzymes.[2] The primary route of elimination is likely by urinary excretion for nusinersen and its metabolites.[2]
Chemistry
Nusinersen is an antisense oligonucleotide in which the 2’-hydroxy groups of the ribofuranosyl rings are replaced with 2’-O-2-methoxyethyl groups and the phosphate linkages are replaced with phosphorothioate linkages.[2][7]
History
Nusinersen was discovered in a collaboration between Adrian Krainer at Cold Spring Harbor Laboratory and Ionis Pharmaceuticals (formerly called Isis Pharmaceuticals).[8][9][10][11] Partial work was done at the University of Massachusetts Medical School funded by Cure SMA.[12]
Starting in 2012, Ionis partnered with Biogen on development and in 2015 Biogen acquired an exclusive license to the drug for a US$75 million license fee, milestone payments up to US$150 million, and tiered royalties thereafter; Biogen also paid the costs of development subsequent to taking the license.[13] The license to Biogen included licenses to intellectual property that Ionis had acquired from Cold Spring Harbor Laboratory and University of Massachusetts.[14]
In November 2016, the new drug application was accepted under the FDA's priority review process on the strength of the Phase III trial and the unmet need, and was also accepted for review at the European Medicines Agency (EMA) at that time.[15][16] It was approved by the FDA in December 2016 and by EMA in May 2017 as the first drug to treat spinal muscular atrophy.[17][18] Subsequently, nusinersen was approved to treat SMA in Canada (July 2017),[19] Japan (July 2017),[20] Brasil (August 2017)[21] and Switzerland (September 2017).[22]
Controversy and access
Spinraza list price is US$125,000 per injection which puts the treatment cost at US$750,000 in the first year and US$375,000 annually after that. According to the New York Times, this places Spinraza "among the most expensive drugs in the world".[16]
In October 2017, the authorities in Denmark recommended Spinraza for use only in a small subset of people with SMA type 1 (young babies) and refused to offer it as a standard treatment for all other people with SMA quoting an "unreasonably high price" compared to the clinical effect.[23] Norwegian authorities rejected the funding in October 2017 because the price of the medicine was "unethically high".[24] In February 2018 the funding was approved for people under 18 years old.[24]
In August 2018 the National Institute for Health and Care Excellence (NICE), the agency that weighs the cost-effectiveness of therapies for the public health system of England and Wales, recommended against offering nusinersen to SMA patients.[25] This decision was met with outrage by the patient advocacy groups.[26]
References
- ^ a b "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 74" (PDF). World Health Organization. pp. 413–14. Retrieved 13 March 2017.
- ^ a b c d e f g h i j k "Nusinersen US Label" (PDF). FDA. December 2016. For updates see FDA index page for NDA 209531
- ^ "Nusinersen". AdisInsight. Retrieved 1 January 2017.
- ^ Ottesen, Eric W. (2017-01-01). "ISS-N1 makes the first FDA-approved drug for spinal muscular atrophy". Translational Neuroscience. 8 (1): 1–6. doi:10.1515/tnsci-2017-0001. ISSN 2081-6936. PMC 5382937. PMID 28400976.
- ^ "Nusinersen". UK Specialist Pharmacy Service. Retrieved 31 December 2016.
- ^ "New warning of nusinersen-related communicating hydrocephalus". Reactions Weekly. 1714 (1): 3–3. 2018-08-01. doi:10.1007/s40278-018-50183-2. ISSN 1179-2051.
- ^ a b Zanetta, C; Nizzardo, M; Simone, C; Monguzzi, E; Bresolin, N; Comi, GP; Corti, S (1 January 2014). "Molecular Therapeutic Strategies for Spinal Muscular Atrophies: Current and Future Clinical Trials". Clinical Therapeutics. 36 (1): 128–40. doi:10.1016/j.clinthera.2013.11.006. PMID 24360800.
- ^ Garber, K (11 October 2016). "Big win possible for Ionis/Biogen antisense drug in muscular atrophy". Nature Biotechnology. 34 (10): 1002–1003. doi:10.1038/nbt1016-1002. PMID 27727217.
- ^ Wadman, Meredith (23 December 2016). "Updated: FDA approves drug that rescues babies with fatal neurodegenerative disease". Science.
- ^ Offord, Catherine (December 1, 2016). "Oligonucleotide Therapeutics Near Approval". The Scientist.
- ^ Tarr, Peter (24 December 2016). "CSHL FDA approval of life-saving SMA drug is hailed by its researcher-inventor at CSHL". Cold Spring Harbor Laboratory.
- ^ "Therapeutic Approaches". www.curesma.org. Cure SMA. Retrieved 1 January 2017.
- ^ "Biogen Shells Out $75M to Develop Ionis' Nusinersen after Positive Phase III Results", Genetic Engineering News, August 1, 2016
- ^ "Press release: Biogen and Ionis Pharmaceuticals Report Nusinersen Meets Primary Endpoint at Interim Analysis of Phase 3 ENDEAR Study in Infantile-Onset Spinal Muscular Atrophy | Biogen Media". Biogen. August 1, 2016.
- ^ "Regulatory Applications for SMA Therapy Nusinersen Accepted in US, EU". BioNews Services, LLC. Retrieved 2016-11-15.
- ^ a b Katie Thomas (December 30, 2016). "Costly Drug for Fatal Muscular Disease Wins F.D.A. Approval". New York Times.
- ^ Grant, Charley (2016-12-27). "Surprise Drug Approval Is Holiday Gift for Biogen". Wall Street Journal. ISSN 0099-9660. Retrieved 2016-12-27.
- ^ "Spinraza (nusinersen)". European Medicines Agency. Retrieved 2017-10-27.
- ^ "Biogen's SPINRAZA™ (nusinersen) Receives Notice of Compliance from Health Canada for the Treatment of 5q Spinal Muscular Atrophy (SMA)". Cision. 2017-07-04.
- ^ "Biogen to launch Spinraza in Japan soon". 2017-07-10.
- ^ "Remédio inédito para atrofia muscular espinhal é liberado" (in Brazilian Portuguese). 2017-08-25.
- ^ "Spinraza – Zulassung nun auch in der Schweiz" (in Swiss High German). SMA Schweiz. 2017-09-30.
- ^ Medicinrådet siger nej til lægemiddel til børn med muskelsvind: 'Urimeligt' dyrt Retrieved October 13 2017.
- ^ a b Dette er uforståelig og utrolig urettferdig
- ^ [1]
- ^ [2]
Further reading
- Finkel, Richard S; Chiriboga, Claudia A; Vajsar, Jiri; Day, John W; Montes, Jacqueline; De Vivo, Darryl C; Yamashita, Mason; Rigo, Frank; Hung, Gene; Schneider, Eugene; Norris, Daniel A; Xia, Shuting; Bennett, C Frank; Bishop, Kathie M (2016). "Treatment of infantile-onset spinal muscular atrophy with nusinersen: A phase 2, open-label, dose-escalation study". The Lancet. 388 (10063): 3017. doi:10.1016/S0140-6736(16)31408-8.
