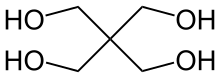
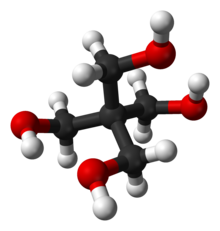
Pentaerythritol
| |
| |
| Names | |
|---|---|
| IUPAC name
2,2-Bis(hydroxymethyl)1,3-propanediol
| |
| Other names
Hercules P 6, monopentaerythritol, tetramethylolmethane, THME, PETP, pentaerythrite, Pentek, Hercules Aqualon improved technical PE-200
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.732 |
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 |
| Appearance | white solid |
| Density | 1.396g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| 5.6 g/100 mL at 15 °C | |
| Solubility | Soluble in methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in acetone, benzene, paraffin, ether, CCl4 |
| Vapor pressure | 0.00000008 mmHg (20°C)[1] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[1] |
IDLH (Immediate danger)
|
N.D.[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pentaerythritol is an organic compound with the formula C5H12O4. This white, crystalline polyol with the neopentane backbone is a versatile building block for the preparation of many polyfunctionalized compounds such as the explosive pentaerythritol tetranitrate and pentaerythritol tetraacrylate.[2] Derivatives of pentaerythritol are components of alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, and olefin antioxidants.
Halogen-free pentaerythritol esters are also environmentally friendly alternative to conventional electrical transformer fluids, being both readily biodegradable and non-hazardous in water. They advantageously replace polychlorobiphenyl (PCB), and even silicone-based or fluorinated hydrocarbons, as dielectric fluid in transformers. Their low volatility and high flash point give them an excellent resistance to ignition in case of major electrical failure and transformer rupture.
Pentaerythritol also finds use in pyrotechnics, as it is needed to make 'blue aluminium'.
Pentaerythritol was first synthesized in 1891 by German chemists Bernhard Tollens and his student P. Wigand.[3]
References
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0485". National Institute for Occupational Safety and Health (NIOSH).
- ^ S. F. Marrian (1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ^ B. Tollens and P.Wigand (1891) "Ueber den Penta-Erythrit, einen aus Formaldehyd und Acetaldehyd synthetisch hergestellten vierwerthigen Alkohol" (On pentaerythritol, a quaternary alcohol synthetically produced from formaldehyde and acetaldehyde), Annalen der Chemie, 265 : 316-340.
