Pericyte
| Pericyte | |
|---|---|
 Pericytes characteristically lining the outer surface of endothelial cells which encircle an erythrocyte (E). | |
| Details | |
| Identifiers | |
| Latin | pericytus |
| MeSH | D020286 |
| TH | H3.09.02.0.02006 |
| FMA | 63174 |
| Anatomical terminology | |
A pericyte is a type of cell found in the central nervous system. Pericytes are more specifically located surrounding the endothelial cell layers of the capillary network in the brain. Pericytes play an integral role in the maintenance of the blood–brain barrier as well as several other homeostatic and hemostatic functions of the brain.[1] These cells are also a key component of the neurovascular unit, which includes endothelial cells, astrocytes, and neurons.[2] Pericytes provide a variety of functions such as capillary blood flow regulation, clearance and phagocytosis of cellular debris, and regulating blood–brain barrier permeability. Pericytes can also work to stabilize and monitor the maturation of endothelial cells by direct communication between the cell membrane as well as paracrine signaling.[3] It has also been revealed in recent studies that a lack of pericytes in the central nervous system can cause a breakdown of the blood–brain barrier and lead to other degenerative changes in the brain.[1]
Morphology
In the central nervous system, pericytes wrap around the endothelial cells that line the outside of the capillary. These two types of cells can be easily distinguished from one another based on the presence of the prominent round nucleus of the pericyte compared to the flat elongated nucleus of the endothelial cells.[2] Pericytes also project finger-like extensions that wrap around the capillary wall, allowing the cells to regulate capillary blood flow.[1] Both pericytes and endothelial cells share a basement membrane where a variety of intercellular connections are made. Many types of integrin molecules facilitate communication between pericytes and endothelial cells separated by the basement membrane.[1] Pericytes can also form direct connections with neighboring cells by forming peg and socket arrangements in which parts of the cells interlock, similar to the gears of a clock. At these interlocking sites, gap junctions can be formed which allow the pericytes and neighboring cells to exchange ions and other small molecules.[1] Important molecules in these intercellular connections include N-cadherin, fibronectin, connexin and various integrins.[2] In some regions of the basement membrane, adhesion plaques composed of fibronectin can be found. These plaques fascilitate the connection of the basement membrane to the cytoskeletal structure composed of actin, and the plasma membrane of the pericytes and endothelial cells.[1]
Function
Blood–brain barrier
Pericytes play a crucial role in the formation and functionality of the selective permeable space between the circulatory system and central nervous system. This space is known as the blood–brain barrier. This barrier is composed of endothelial cells and assures the protection and functionality of the brain and central nervous system. Although it had been theorized that astrocytes were crucial to the postnatal formation of this barrier, it has been found that pericytes are now largely responsible for this role. Pericytes are responsible for tight junction formation and vesicle trafficking amongst endothelial cells. Furthermore, they allow the formation of the blood–brain barrier by inhibiting the effects of CNS immune cells (which can damage the formation of the barrier) and by reducing the expression of molecules that increase vascular permeability.[4]
Aside from blood–brain barrier formation, pericytes also play an active role in its functionality by controlling the flow within blood vessels and between blood vessels and the brain. As contractile cells, they can open or close a given amount to allow (or disallow) certain sized particles to flow through the vessel. Such regulation of blood flow is beneficial to neuronal function because it prevents certain particles in the blood from entering the brain. When pericytes are not present, a process known as transcytosis occurs in the blood–brain barrier. This essentially allows particles of varying sizes, including large plasma proteins, to easily enter the brain with little to no regulation. This process is peculiar because the blood–brain barrier is highly regulatory under normal conditions. Therefore, such may confer a dysfunction of pericytes in the blood–brain barrier.[5] Thus, pericytes play a critical role in assuring that harmful chemicals do not enter the brain and disrupt neurological function. Pericyte functionality (or disfunctionality) is also theorized to contribute to neurodegenerative diseases such as Alzheimer’s, Parkinson’s and ALS (Lou Gehrig's Disease). Furthermore, the elasticity of pericyte is beneficial because they can expand to reduce inflammation and allow harmful substances to diffuse out of the brain.[6]
These cells also play a key role in increasing microcirculation and reducing the effects of brain aging. In a study involving adult pericyte-deficient mice, the absence of such cells in the brain had been found to lead to vascular damage from loss of microcirculation and cerebral blood flow. Such blood flow is imperative to mediate the effects caused by stress, hypoxia and several other conditions which may alter homeostasis. In addition, when pericytes are not present, the blood–brain barrier does not degrade certain neurotoxic and vasculotoxic serum proteins which thus bolsters degenerative changes. Such changes include inflammation as well as learning and memory impairment.[7]
Angiogenesis and the survival of endothelial cells
Pericytes are also associated with allowing endothelial cells to differentiate, multiply, form vascular branches (Angiogenesis), survive apoptotic signals and travel throughout the body. Certain pericytes, known as microvascular pericytes develop around the walls of capillaries and help to serve this function. Microvascular pericytes may not be contractile cells because they lack alpha-actin isoforms; structures that are common amongst other contractile cells. These cells communicate with endothelial cells via gap junctions and in turn cause endothelial cells to proliferate or be selectively inhibited. If this process did not occur, hyperplasia and abnormal vascular morphogenesis could occur. These types of pericyte can also phagocytose exogenous proteins. This suggests that the cell type might have been derived from microglia.[8]
It is also important to note that pericytes maintain plasticity and thus can differentiate into various other cell types including, smooth muscle cells as well as fibroblasts and other mesenchymal stem cells. Such versatility is conducive because they actively remodel blood vessels throughout the body and can thereby blend homogeneously with the local tissue environment .[9]
Aside from creating and remodeling blood vessels in a viable fashion, pericytes have been found to protect endothelial cells from death via apoptosis or cytotoxic elements. It has been studied in vivo that pericytes release a hormone known as pericytic aminopeptidase N/pAPN that may help to promote angiogenesis. When this hormone was mixed with cerebral endothelial cells as well as astrocytes, the pericytes grouped into structures that resembled capillaries. Furthermore, if experimental group contained all of the following with the exception of pericytes, the endothelial cells would undergo apoptosis. That being said, it was concluded that pericytes must be present to assure the proper function of endothelial cells and astrocytes must be present to assure that both remain in contact. If not, than proper angiogenesis cannot occur.[10] In addition, it has been found that pericytes contribute to the survival of endothelial cells because they secrete the protein Bcl-w during cellular crosstalk. Bcl-w is an instrumental protein in the pathway that enforces VEGF-A expression and discourages apoptosis.[11] Although there is some speculation of why VEGF is directly responsible fro preventing apoptosis, it is believed to be responsible for modulating apoptotic signal transduction pathways and inhibiting activation of apoptosis inducing enzymes. Two biochemical mechanisms utilized by VEGF to accomplish such would be phosphorylation of extracellular regulatory kinase 1 (ERK-1) which sustains cell survival over time and inhibition of stress-activated protein kinase/c-jun-NH2 kinase which also promotes apoptosis.[12]
Pathologies
Because of their crucial role in maintaining and regulating endothelial cell structure and blood flow, abnormalities in pericyte function are seen in many pathologies. They may either be present in excess, leading to diseases such as hypertension and tumor formation, or in deficiency, leading to neurodegenerative diseases.
Hemangiopericytoma
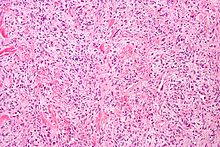
Hemangiopericytoma is a rare vascular neoplasm, or abnormal growth, that may either be benign or malignant. In its malignant form, metastasis to the lungs, liver, brain, and extremities may occur. It most commonly manifests itself in the femur and proximal tibia as a bone sarcoma, and is usually found in older individuals, though cases have been found in children. Hemangiopericytoma is caused by the excessive layering of sheets of pericytes around improperly formed blood vessels. Diagnosis of this tumor is difficult because of the inability to distinguish pericytes from other types of cells using light microscopy. Treatment may involve surgical removal and radiation therapy, depending on the level of bone penetration and stage in the tumor's development.[13]
Diabetic retinopathy
The retina of diabetic individuals often exhibits loss of perictyes, and this loss is a characteristic factor of the early stages of diabetic retinopathy. Studies have found that pericytes are essential in diabetic individuals to protect the endothelial cells of retinal capillaries. With the loss of perictyes, microaneuryisms form in the capillaries. In response, the retina either increases its vascular permeability, leading to swelling of the eye through a macular edema, or forms new vessels that permeate into the vitreous membrane of the eye. The end result is reduction or loss of vision.[14] While it is unclear why pericytes are lost in diabetic patients, one hypothesis is that toxic sorbitol and advanced glycation end products (AGE) accumulate in the pericytes. Because of the build-up of glucose, the polyol pathway increases its flux, and intracellular sorbitol and fructose accumulate. This leads to osmotic imbalance,which results in cellular damage. The presence of high glucose levels also leads to the buildup of AGE's, which also damage cells. [15]
Neurodegenerative diseases
Studies have found that pericyte loss in the adult and aging brain leads to the disruption of proper cerebral perfusion and maintenance of the blood–brain barrier, which causes neurodegeneration and neuroinflammation. The apoptosis of pericytes in the aging brain may be the result of a failure in communication between growth factors and receptors on pericytes. Platelet-derived growth factor B (PDGF-B)is released from endothelial cells in brain vasculature and binds to the receptor PDGFR-Beta on pericytes, initiating their proliferation and migration for proper vasculature maintenance. When this signalling is disrupted, pericytes undergo apoptosis, leading to many neurodegenerative diseases including Alzheimer's disease and Multiple Sclerosis.[16]
Current research
Endothelial and pericyte interactions
Endothelial cell and pericytes are interdependent, so failure of proper communication between the two cells can lead to numerous human pathologies, such as the ones listed above.[17]
There are several pathways of communication between the endothelial cells and pericytes. The first is transforming growth factor (TGF) signaling, which is mediated by endothelial cells. This is important for pericyte differentiation.[18][19] Angiopoietin 1 and Tie-2 signaling is essential for maturation and stabilization of endothelial cells.[20] Platelet-derived growth factor (PDGF) pathway signaling from endothelial cells recruit pericytes, so that pericytes can migrate to growing vessels. If this pathway is blocked, it leads to pericyte deficiency.[21] Sphingosine-1-phosphate (S1P) signaling also aides in pericyte recruitment by communication through G protein-coupled receptors. S1P signals through GTPases that promote N-cadherin trafficking to endothelial membranes. This trafficking strengthens contacts with pericytes.[22]
Communication between endothelial cells and pericytes are important. Inhibiting the PDGF pathway leads to pericyte deficiency. This causes endothelial hyperplasia, abnormal junctions, and diabetic retinotropy.[14] A lack of pericytes also causes an upregulation of vascular endothelial growth factor (VEGF), leading to vascular leakage and hemorrhage.[23] Also, angiopoietin 2 can act as an antagonist to Tie-2.[24] This destabilizes the endothelial cells, which accounts for less endothelial cell and pericyte interaction. This can actually lead to the formation of tumors.[25] Similar to the inhibition of the PDGF pathway, angiopoietin 2 reduces levels of pericyte, leading to diabetic retinopathy.[26]
Scarring
After an injury in the CNS, scarring occurs to preserve the integrity of surrounding cells. Usually, astrocytes are associated with the scarring and are called glial scars. However, there is a stromal or nonglial component of the scarring, and there is evidence that perivascular pericytes play a critical role in assisting scar formation.[27]
An experiment was conducted on mice that have been genetically labeled using glutamate aspartate transporter (Glast). They found two different subtypes of pericytes and termed them subtype A and B. Subtype A accounts for about 10% of the pericytes in the adult spinal cord and plays a critical role in scarring. Subtype B accounts for the rest of the pericytes and has desmin and alpha smooth muscles. After the two subtypes were isolated and labeled, an incision was made to the dorsal part of the brain. Typically in an uninjured spinal cord there are ten times as many astrocytes than subtype A pericytes.[28] However, two weeks after the injury, the number of pericytes were double that of the astrocytes. Subtype A were three times more prevalent than subtype B. The main difference in prevalence arises from the fact that subtype A is able to detach from blood vessel walls and can send tiny projections to nearby cells and deposit extracellular matrix proteins.
The scarring is highly compartmentalized. The pericytes form the core of the scar, while ependymal cells form a second layer around the core, followed by another layer of astrocytes that originated through self-duplication.[28]
Inhibition of subtype A pericyte generation caused improper closing of spinal cord incisions, which supports the idea that pericytes are important for scarring.
See also
- Hemangiopericytoma
- Mural cell
- Mesoangioblast
- Diabetic retinopathy caused by death of pericytes
References
- ^ a b c d e f Winkler, EA.; Bell, RD.; Zlokovic, BV. (2011). "Central nervous system pericytes in health and disease". Nat Neurosci. 14 (11): 1398–405. doi:10.1038/nn.2946. PMID 22030551.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ a b c Dore-Duffy, P.; Cleary, K. (2011). "Morphology and properties of pericytes". Methods Mol Biol. 686: 49–68. doi:10.1007/978-1-60761-938-3_2. PMID 21082366.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Fakhrejahani, E.; Toi, M. (2012). "Tumor angiogenesis: pericytes and maturation are not to be ignored". J Oncol. 2012: 261750. doi:10.1155/2012/261750. PMID 22007214.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: unflagged free DOI (link) - ^ Daneman, Richard et al. (2010) Pericytes are required for blood–brain barrier integrity during embryogenesis. "nature" 468, 562-566
- ^ Investigators Reveal Key to Blood-Brain Barrier. This passage of particles can be adverse to brain function because many of such can are toxic or harmful to some extent."GEN" (2010)http://www.genengnews.com/gen-news-highlights/investigators-reveal-key-to-blood-brain-barrier/81244067/
- ^ Pericytes regulate blood-brain barrier (2010) [1]
- ^ Bell, Robert D. et al. Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging. "Cell" 68 Vol 3 409-427 (2010) http://www.cell.com/neuron/abstract/S0896-6273(10)00824-X
- ^ Pericyte, Astrocyte and Basal Lamina Association with the Blood Brain Barrier (BBB) http://davislab.med.arizona.edu/content/pericyte-astrocyte-and-basal-lamina-association-blood-brain-barrier-bbb
- ^ Gerhardt H, Betsholtz C.(2003) Endothelial-pericyte interactions in angiogenesis. "Cell Tissue Res." 314(1), 15-23 http://www.ncbi.nlm.nih.gov/pubmed/12883993
- ^ Ramsauer, Markus et. al. (2002) Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes "The FASEB Journal" 10.1096/fj.01-0814fje http://www.fasebj.org/content/early/2002/08/02/fj.01-0814fje.full.pdf
- ^ Franco, Marcela et al. (2011) Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression "Blood" http://bloodjournal.hematologylibrary.org/content/118/10/2906
- ^ VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999 Mar 15;247(2):495-504. http://www.ncbi.nlm.nih.gov/pubmed/10066377
- ^ Gellman, Harris. "Medscape: Medscape Access". Retrieved 2 November 2011.
- ^ a b Hammes, HP.; Lin, J.; Renner, O.; Shani, M.; Lundqvist, A.; Betsholtz, C.; Brownlee, M.; Deutsch, U. (2002). "Pericytes and the pathogenesis of diabetic retinopathy". Diabetes. 51 (10): 3107–12. PMID 12351455.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Ciulla, TA.; Amador, AG.; Zinman, B. (2003). "Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies". Diabetes Care. 26 (9): 2653–64. PMID 12941734.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bell, Robert D.; Winkler, Abhay P. Sagare; Singh, Itender; LaRue, Barb; Deane, Rashid; Zlokovic, Berislav V. (2010). "Pericytes Control Key Neurovascular Functions and Neuronal Phenotype in the Adult Brain and during Brain Aging". Neuron. 68 (3): 409–27.
{{cite journal}}: Unknown parameter|month=ignored (help)http://www.cell.com/neuron/abstract/S0896-6273(10)00824-X - ^ Armulik, A.; Abramsson, A.; Betsholtz, C. (2005). "Endothelial/pericyte interactions". Circ Res. 97 (6): 512–23. doi:10.1161/01.RES.0000182903.16652.d7. PMID 16166562.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Carvalho, RL.; Jonker, L.; Goumans, MJ.; Larsson, J.; Bouwman, P.; Karlsson, S.; Dijke, PT.; Arthur, HM.; Mummery, CL. (2004). "Defective paracrine signalling by TGFbeta in yolk sac vasculature of endoglin mutant mice: a paradigm for hereditary haemorrhagic telangiectasia". Development. 131 (24): 6237–47. doi:10.1242/dev.01529. PMID 15548578.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hirschi, KK.; Rohovsky, SA.; D'Amore, PA. (1998). "PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate". J Cell Biol. 141 (3): 805–14. PMID 9566978.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thurston, G.; Suri, C.; Smith, K.; McClain, J.; Sato, TN.; Yancopoulos, GD.; McDonald, DM. (1999). "Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1". Science. 286 (5449): 2511–4. doi:10.1126/science.286.5449.2511. PMID 10617467.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bjarnegård, M.; Enge, M.; Norlin, J.; Gustafsdottir, S.; Fredriksson, S.; Abramsson, A.; Takemoto, M.; Gustafsson, E.; Fässler, R. (2004). "Endothelium-specific ablation of PDGFB leads to pericyte loss and glomerular, cardiac and placental abnormalities". Development. 131 (8): 1847–57. doi:10.1242/dev.01080. PMID 15084468.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Paik, JH.; Skoura, A.; Chae, SS.; Cowan, AE.; Han, DK.; Proia, RL.; Hla, T. (2004). "Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization". Genes Dev. 18 (19): 2392–403. doi:10.1101/gad.1227804. PMID 15371328.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hellström, M.; Gerhardt, H.; Kalén, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. (2001). "Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis". J Cell Biol. 153 (3): 543–53. PMID 11331305.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Maisonpierre, PC.; Suri, C.; Jones, PF.; Bartunkova, S.; Wiegand, SJ.; Radziejewski, C.; Compton, D.; McClain, J.; Aldrich, TH. (1997). "Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis". Science. 277 (5322): 55–60. doi:10.1126/science.277.5322.55. PMID 9204896.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Zhang, L.; Yang, N.; Park, JW.; Katsaros, D.; Fracchioli, S.; Cao, G.; O'Brien-Jenkins, A.; Randall, TC.; Rubin, SC. (2003). "Tumor-derived vascular endothelial growth factor up-regulates angiopoietin-2 in host endothelium and destabilizes host vasculature, supporting angiogenesis in ovarian cancer". Cancer Res. 63 (12): 3403–12. PMID 12810677.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hammes, HP.; Lin, J.; Wagner, P.; Feng, Y.; Vom Hagen, F.; Krzizok, T.; Renner, O.; Breier, G.; Brownlee, M. (2004). "Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy". Diabetes. 53 (4): 1104–10. PMID 15047628.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Göritz, C.; Dias, DO.; Tomilin, N.; Barbacid, M.; Shupliakov, O.; Frisén, J. (2011). "A pericyte origin of spinal cord scar tissue". Science. 333 (6039): 238–42. doi:10.1126/science.1203165. PMID 21737741.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, FW.; Meletis, K.; Frisén, J. (2010). "Origin of new glial cells in intact and injured adult spinal cord". Cell Stem Cell. 7 (4): 470–82. doi:10.1016/j.stem.2010.07.014. PMID 20887953.
{{cite journal}}: Unknown parameter|month=ignored (help)
External links
- Pericytes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Diagram at udel.edu
