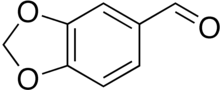
Piperonal
| |
| Names | |
|---|---|
| Preferred IUPAC name
2H-1,3-Benzodioxole-5-carbaldehyde | |
| Other names
Heliotropin; Heliotropine; Piperonyl aldehyde; Protocatechuic aldehyde methylene ether; 3,4-methylenedioxybenzaldehyde;
| |
| Identifiers | |
3D model (JSmol)
|
|
| 131691 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.009 |
| EC Number |
|
| 4186 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H6O3 | |
| Molar mass | 150.133 g·mol−1 |
| Appearance | Colorless crystals[1] |
| Density | 1.337 g/cm3 |
| Melting point | 37 °C (99 °F; 310 K)[1] |
| Boiling point | 263 °C (505 °F; 536 K)[1] |
| Soluble in 500 parts[1] | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H317 | |
| P261, P272, P280, P302+P352, P321, P333+P313, P363, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2700 mg/kg (orally in rats)[1] |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors.[3] The molecule is structurally related to other aromatic aldehydes such as benzaldehyde and vanillin.
Natural occurrence
[edit]Piperonal naturally occurs in various plants. Examples include dill, vanilla, violet flowers, and black pepper.
Preparation
[edit]Piperonal can be prepared by the oxidative cleavage of isosafrole or by using a multistep sequence from catechol or 1,2-methylenedioxybenzene. Synthesis from the latter chemical is accomplished through a condensation reaction with glyoxylic acid followed by cleaving the resulting α-hydroxy acid with an oxidizing agent.[3][4][5] Synthesis from catechol requires an additional step, Williamson ether synthesis using dichloromethane.[6]
Reactions
[edit]Piperonal, like all aldehydes, can be reduced to its alcohol (piperonyl alcohol) or oxidized to give its acid (piperonylic acid).
Piperonal can be used in the synthesis of some pharmaceutical drugs including tadalafil,[7] L-DOPA,[8] and atrasentan.[9]
Fragrance
[edit]Piperonal has a floral odor which is commonly described as being similar to that of vanillin or cherry. For this reason it is commonly used in fragrances and artificial flavors.[3] The compound was named heliotropin after the 'cherry pie' notes found in the heliotrope flower's fragrance (even though the chemical is not present in the flower's true aroma).[10] Perfumers began to use the fragrance for the first time by the early 1880s.[11] It is commonly used to add vanilla or almond nuances, generally imparting balsamic, powdery, and floral aspects to a scent's character.[12]
Piperonyl acetate is a synthetic cherry flavoring.[13]
Use in MDMA manufacture
[edit]Due to their role in the manufacture of MDMA, safrole, isosafrole, and piperonal are Category I precursors under regulation no. 273/2004 of the European Community.[14]
References
[edit]- ^ a b c d e Budavari, Susan, ed. (1996), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- ^ a b c Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe and Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2003. doi:10.1002/14356007.a11_141
- ^ Fatiadi, Alexander & Schaffer, Robert (1974). "An Improved Procedure for Synthesis of DL-4-Hydroxy-3-methoxymandelic Acid (DL-"Vanillyl"-mandelic Acid, VMA)". Journal of Research of the National Bureau of Standards Section A. 78A (3): 411–412. doi:10.6028/jres.078A.024. PMC 6742820. PMID 32189791.
- ^ Nwaukwa, Stephen; Keehn, Philip (1982). "Oxidative cleavage of α-diols, α-diones, α-hydroxy-ketones and α-hydroxy- and α-keto acids with calcium hypochlorite [Ca(OCl)2]". Tetrahedron Letters. 23 (31): 3135–3138. doi:10.1016/S0040-4039(00)88578-0.
- ^ Bonthrone, W. & Cornforth, J. (1969). "The methylenation of catechols". Journal of the Chemical Society (9): 1202–1204. doi:10.1039/J39690001202.
- ^ Gilla, G.; Anumula, R.R.; Aalla, S.; Vurimidi, H. & Ghanta, M.R. (2013). "Synthesis and characterization of related substances and metabolite of tadalafil, a PDE-5 inhibitor" (PDF). Organic Communications. 6 (1): 12–22. Archived (PDF) from the original on 2018-10-24. Retrieved 2015-01-21.
- ^ Yamada, Shun-Ichi; Fujii, Tozo; Shioiri, Takayuki (1962). "Studies on Optically Active Amino Acids. I. Preparation of 3-(3, 4-Methylenedioxyphenyl)-D-, and -L-alanine". Chemical & Pharmaceutical Bulletin. 10 (8): 680–688. doi:10.1248/cpb.10.680. PMID 14002245.
- ^ Winn, Martin; von Geldern, Thomas W.; Opgenorth, Terry J.; Jae, Hwan-Soo; Tasker, Andrew S.; Boyd, Steven A.; Kester, Jeffrey A.; Mantei, Robert A.; Bal, Radhika; Sorensen, Bryan K.; Wu-Wong, Jinshyun R.; Chiou, William J.; Dixon, Douglas B.; Novosad, Eugene I.; Hernandez, Lisa; Marsh, Kennan C. (1996). "2,4-Diarylpyrrolidine-3-carboxylic AcidsPotent ETASelective Endothelin Receptor Antagonists. 1. Discovery of A-127722". Journal of Medicinal Chemistry. 39 (5): 1039–1048. doi:10.1021/jm9505369. ISSN 0022-2623. PMID 8676339.
- ^ "Essential oils". Archived from the original on 2019-12-23. Retrieved 2012-09-02.
- ^ The Force of Fashion in Politics and Society: Global Perspectives from Early Modern to Contemporary Times By Beverly Lemire ISBN 9781409404927
- ^ The Good Scents Company database entry for Heliotropin Archived 2020-08-01 at the Wayback Machine
- ^ Fenaroli's Handbook of Flavor Ingredients.
- ^ Regulation (EC) No 273/2004 of the European Parliament and of the Council of 11 February 2004 on drug precursors
