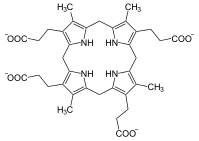
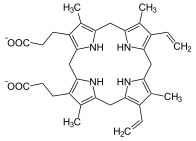
Porphyrinogen
In biochemistry a porphyrinogen is a member of a class of naturally occurring tetrapyrrole macrocycles.[1] Porphyrinogens are intermediates in the biosynthesis of porphyrin-containing cofactors, which are found in many enzymes and proteins including myoglobin, hemoglobin, cytochromes, and chlorophylls.[2]
Porphyrinogens have four methylene groups and four pyrrole rings. In porphyrins, the four C4N rings are linked by methyne (CH) groups, and two of the C4N rings have no NH groups:
- porphyrinogen → porphyrin + 3 "H2"
In the biosynthesis of porphyrins protoporphyrinogen is dehydrogenated by protoporphyrinogen oxidase.
Because of their limited delocalization, porphyrinogens are colorless. Subsequent biosynthetic intermediates en route to porphyrins are deeply colored and often phytotoxic.
- Naturally occurring porphyrinogens
-
Uroporphyrinogen III, precursor to coproporphyrinogen III.
-
coproporphyrinogen III, precursor to protoporphyrinogen IX.
-
Protoporphyrinogen IX, precursor to protoporphyrin IX.
Non-natural porphyrinogens
Porphyrinogens are intermediates in the so-called Lindsey synthesis of meso-substituted porphyrins. Thus under acid catalysis, pyrrole and aldehydes condense to give the porphyrinogens of the type (R(H)CC4H2NH)n including the tetramer (n = 4). Subsequent oxidation of these reduced macrocycles delivers the porphyrin.[3]
Porphyrinogens bearing eight organic substituents at the meso positions are also called calix[4]pyrroles. Upon tetradeprotonation, they are as ligands in coordination chemistry. One example is octaethyporphyrinogen, derived from the condensation of pyrrole and diethylketone. The octasubstituted porphyrinogens resist dehydrogenation.[4]

References
- ^ porphyrinogens - IUPAC Gold Book
- ^ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.
- ^ Lindsey, Jonathan S. (2000). "Synthesis of meso-substituted porphyrins". Porphyrin Handbook. Vol. 1. pp. 45–118. ISBN 0-12-393200-9.
{{cite encyclopedia}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Sessler, Jonathan L.; Anzenbacher, Pavel, Jr.; Miyaji, Hidekazu; Jursikova, Karolina; Bleasdale, Ellen R.; Gale, Philip A. (2000). "Modified Calix[4]pyrroles". Industrial & Engineering Chemistry Research. 39: 3471–3478. doi:10.1021/ie000102y.
{{cite journal}}: CS1 maint: multiple names: authors list (link)