Potassium bitartrate
This article needs additional citations for verification. (April 2011) |
| |
| Names | |
|---|---|
| Other names
potassium hydrogen tartrate
cream of tartar potassium acid tartrate monopotassium tartrate | |
| Identifiers | |
| ECHA InfoCard | 100.011.609 |
| E number | E336 (antioxidants, ...) |
| Properties | |
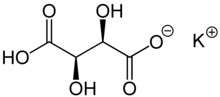
| KC4H5O6 | |
| Molar mass | 188.177 |
| Appearance | white crystalline powder |
| Density | 1.05 g/cm3 (solid) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium bitartrate, also known as potassium hydrogen tartrate, has formula KC4H5O6, is a byproduct of winemaking. In cooking it is known as cream of tartar. It is the potassium acid salt of tartaric acid, a carboxylic acid.
Occurrence
Potassium bitartrate crystallizes in wine casks during the fermentation of grape juice, and can precipitate out of wine in bottles. The crystals (wine diamonds) will often form on the underside of a cork in wine-filled bottles that have been stored at temperatures below 10 °C (50 °F), and will seldom, if ever, dissolve naturally into the wine.
These crystals also precipitate out of fresh grape juice that has been chilled or allowed to stand for some time.[1] To prevent crystals forming in homemade grape jam or jelly, fresh grape juice should be chilled overnight to promote crystallisation. The potassium bitartrate crystals are removed by filtering through two layers of cheesecloth; the filtered juice may then be made into jam or jelly.[2] In some cases they adhere to the side of the chilled container, making filtering unnecessary.
The crude form (known as beeswing) is collected and purified to produce the white, odorless, acidic powder used for many culinary and other household purposes.
Applications
In food

In food, potassium bitartrate is used for:
- Stabilising egg whites, increasing their heat tolerance and volume
- Preventing sugar syrups from crystallising
- Reducing discolouration of boiled vegetables
- keeping heat in cereal bars
Additionally it is used as a component of:
- Baking powder, as an acid ingredient to activate baking soda
- Sodium-free salt substitutes, in combination with potassium chloride
A similar acid salt, sodium acid pyrophosphate, can be confused with cream of tartar because of their common function as a component of baking powder.
Household use
Potassium bitartrate can be mixed with an acidic liquid such as lemon juice or white vinegar to make a paste-like cleaning agent for metals such as brass, aluminum or copper, or with water for other cleaning applications such as removing light stains from porcelain. [3] This mixture is sometimes mistakenly made with vinegar and sodium bicarbonate (baking soda), which actually react to neutralise each other, creating carbon dioxide and a sodium acetate solution.
Cream of tartar was often used in traditional dyeing where the complexing action of the tartrate ions were used to adjust the solubility and hydrolysis of mordant salts such as tin chloride and alum.
It is a common ingredient in Play-Doh.[4]
Chemistry
Potassium acid tartrate, also known as potassium hydrogen tartrate, is, according to NIST, used as a primary reference standard for a pH buffer. Using an excess of the salt in water, a saturated solution is created with a pH of 3.557 at 25 °C. Upon dissolution in water, potassium bitartrate will dissociate into acid tartrate, tartrate, and potassium ions. Thus, a saturated solution creates a buffer with standard pH. Before use as a standard, it is recommended that the solution be filtered or decanted between 22 °C and 28 °C.[5]
Potassium carbonate can be made by igniting cream of tartar producing "pearl ash". This process is now obsolete but produced a higher quality (reasonable purity) than "potash" extracted from wood or other plant ashes.
See also
- Tartrate
- Tartaric acid
- Potassium tartrate (K2C4H4O6)
References
- ^ Lloyds Vinyard FAQs
- ^ National Center for Home Food Preservation
- ^ Michigan State University Extension Home Maintenance And Repair - Homemade Cleaners - 01500631, 06/24/03
- ^ Play Dough Recipes
- ^ Harris, Daniel C. (17 July 2006), Quantitative Chemical Analysis (7th ed.), New York: W. H. Freeman, ISBN 978-0716776949
External links
 This article incorporates text from a publication now in the public domain: Ward, Artemas (1911). "The Grocer's Encyclopedia". The Grocer's Encyclopedia.
This article incorporates text from a publication now in the public domain: Ward, Artemas (1911). "The Grocer's Encyclopedia". The Grocer's Encyclopedia.
