Protoporphyrinogen IX
Appearance

| |
| Identifiers | |
|---|---|
| MeSH | protoporphyrinogen |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C34H38N4O4 | |
| Molar mass | 566.7 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
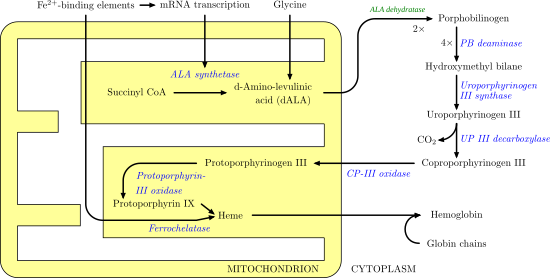
Protoporphyrinogen IX is a precursor to protoporphyrin IX, which gives rise to hemes.
Biosynthetic context
The enzyme coproporphyrinogen oxidase converts coproporphyrinogen III into protoporphyrinogen IX. The process entails conversion of two of four propionic acid groups to vinyl groups. By the action of protoporphyrinogen oxidase, protoporphyrinogen IX is converted into protoporphyrin IX, the first colored tetrapyrrole in the biosynthesis of hemes.[1]
In coproporphyrinogen III, the pyrrole substituents have the arrangement MeP-MeP-MeP-PMe, where Me and P are methyl and propionic, respectively. In protoporphyrinogen IX, the sequence becomes MeV-MeV-MeP-PMe, where V is vinyl.
References
- ^ Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.
See also
