Schiff base


A Schiff base , named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen.[1] Schiff bases in a broad sense have the general formula R1R2C=NR3, where R is an organic side chain. In this definition, Schiff base is synonymous with azomethine. Some restrict the term to the secondary aldimines (azomethines where the carbon is connected to a hydrogen atom), thus with the general formula RCH=NR'.[2]
The chain on the nitrogen makes the Schiff base a stable imine. A Schiff base derived from an aniline, where R3 is a phenyl or a substituted phenyl, can be called an anil.[3]
Synthesis
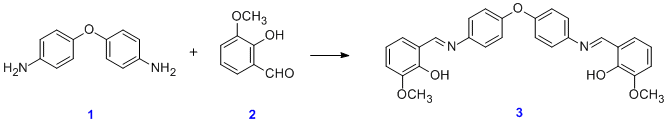
Schiff bases can be synthesized from an aromatic amine and a carbonyl compound by nucleophilic addition forming a hemiaminal, followed by a dehydration to generate an imine. In a typical reaction, 4,4'-diaminodiphenyl ether reacts with o-vanillin:[4]
There is a Schiff base intermediate in the fructose 1,6-bisphosphate aldolase catalyzed reaction during glycolysis.
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff base". doi:10.1351/goldbook.S05498
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "azomethines". doi:10.1351/goldbook.A00564
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "anils". doi:10.1351/goldbook.A00357
- ^ {{Cite journal| last = Jarrahpour| first = A. A.| coauthors = M. Zarei| title = Synthesis of 2-({[4-(4-{[(E)-1-(2-hydroxy-3-methoxyphenyl)methylidene amino}phenoxy)phenyl imino}methyl)- 6 -methoxy phenol| journal = Molbank| volume = M352| date = February 24, 2004| url = http://www.mdpi.net/molbank/molbank2004/m0352.htm%7C issn =1422-8599| accessdate = February 22, 2010}}
