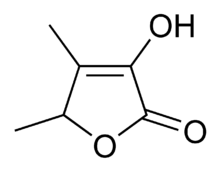
Sotolon
| |
| Names | |
|---|---|
| IUPAC name
3-Hydroxy-4,5-dimethylfuran-2(5H)-one
| |
| Other names
Sotolone
Caramel furanone Sugar lactone Fenugreek lactone | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.044.655 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H8O3 | |
| Molar mass | 128.13 g/mol |
| Density | 1.049 g/cm3 |
| Boiling point | 184 °C (363 °F; 457 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sotolon (also known as sotolone) is a lactone and an extremely powerful aroma compound, with the typical smell of fenugreek or curry at high concentrations and maple syrup, caramel, or burnt sugar at lower concentrations. Sotolon is the major aroma and flavor component of fenugreek seed and lovage,[1] and is one of several aromatic and flavor components of artificial maple syrup.[2] It is also present in molasses, aged rum, aged sake and white wine, flor sherry, roast tobacco,[3] and dried fruiting bodies of the mushroom Lactarius helvus.[4] Sotolon can pass through the body relatively unchanged, and consumption of foods high in sotolon, such as fenugreek, can impart a maple syrup aroma to one's sweat and urine. In some individuals with the genetic disorder maple syrup urine disease, it is spontaneously produced in their bodies and excreted it in their urine, leading to the disease's characteristic smell.[5]
This molecule is thought to be responsible for the mysterious maple syrup smell that has occasionally wafted over Manhattan since 2005.[6]
References
- ^ Imre Blank, Peter Schieberle (1993). "Analysis of the seasoning-like flavour substances of a commercial lovage extract" (abstract). Flavour and Fragrance Journal. 8: 191–195. doi:10.1002/ffj.2730080405.
- ^ Caracteristiques chemiques et nutritives du sirop d'erable
- ^ Sigma-Aldrich
- ^ Sylvie Rapior,a Françoise Fons,a and Jean-Marie Bessièreb (2000). "The fenugreek odor of Lactarius helvus" (abstract). Mycologia. 92 (2). Mycological Society of America: 305–308. doi:10.2307/3761565. JSTOR 3761565.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ F. Podebrad, M. Heil, S. Reichert1, A. Mosandl, A. C. Sewell and H. Böhles (1999). "4,5-Dimethyl-3-hydroxy-2[5H]-furanone (sotolone) — The odour of maple syrup urine disease". Journal of Inherited Metabolic Disease. 22 (2): 107–114. doi:10.1023/A:1005433516026. PMID 10234605.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ [1]
