Sulfamic acid

| |
| Names | |
|---|---|
| IUPAC name
Sulfamic acid
| |
| Identifiers | |
| ECHA InfoCard | 100.023.835 |
| EC Number |
|
| RTECS number |
|
| UN number | 2967 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| H2NSO3H | |
| Molar mass | 97.10 g/mol |
| Density | 2.15 g/cm3 |
| Melting point | 205 °C decomp. |
| moderate, with slow hydrolysis | |
| Related compounds | |
Other cations
|
Ammonium sulfamate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colorless, water-soluble compound finds many applications.
Sulfamic acid (H3NSO3) may be considered an intermediate compound between sulfuric acid (H2SO4), and sulfamide (H4N2SO2), effectively - though see below - replacing an -OH group with an -NH2 group at each step. This pattern can extend no further in either direction without breaking down the -SO2 group.
Structure and reactivity
First, it should be noticed that the compound is well described by the formula H3NSO3, not the tautomer H2NSO2(OH). The relevant bond distances are S=O, 1.44 and S-N 1.77 Å. The greater length of the S-N distance is consistent with a single bond.[1] Furthermore, a neutron diffraction study located the hydrogen atoms, all three of which are 1.03 Å distant from nitrogen[2]. In the solid state, the molecule of sulfamic acid is well described by a zwitterionic form :
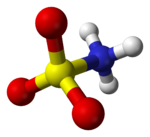 |
acid zwitterion in the crystal[2] |
Sulfamic acid is a moderately strong acid, Ka = 1.01 x 10−1. Because the solid is non-hygroscopic, it is used as a standard in acidometry (quantitative assays of acid content). Double deprotonation can be effected in NH3 solution to give [HNSO3]2−.
Sulfamic acid melts at 205 °C before decomposing at higher temperatures to H2O, SO3, SO2, and N2.
Water solutions are unstable and slowly hydrolyze to ammonium bisulfate, but the crystalline solid is indefinitely stable under ordinary storage conditions.
With HNO2, sulfamic acid reacts to give N2, while with HNO3, it affords N2O.
The behavior of H3NSO3 resembles that of urea, (H2N)2CO, in some ways. Both feature amino groups linked to electron-withdrawing centers that can participate in delocalized bonding. Both liberate ammonia upon heating in water.
Applications
The most famous application of sulfamic acid is in the synthesis of compounds that taste sweet. Reaction with cyclohexylamine followed by addition of NaOH gives C6H11NHSO3Na, sodium cyclamate. Related compounds are also sweeteners, see acesulfame potassium.
Sulfamates (O-substituted-, N-substituted-, or di-/tri-substituted derivatives of sulfamic acid) have been used in the design of many types of therapeutic agents such as antibiotics, nucleoside/nucleotide human immunodeficiency virus (HIV) reverse transcriptase inhibitors, HIV protease inhibitors (PIs), anti-cancer drugs (steroid sulfatase and carbonic anhydrase inhibitors), anti-epileptic drugs, and weight loss drugs.
Sulfamic acid is used as an acidic cleaning agent, sometimes pure or as a component of proprietary mixtures, typically for metals and ceramics. It is frequently used for removing rust and limescale, replacing the more volatile and irritating hydrochloric acid. It is often a component of household descaling agents or detergents used for removal of limescale. When compared to most of the common strong mineral acids, Sulfamic acid has desirable water descaling properties, low volatility, low toxicity and is a water soluble solid forming soluble calcium and iron-III salts. Its also finds applications in the industrial cleaning of dairy and brew-house equipment. Although it is considered less corrosive than hydrochloric acid due to its lower pKa, corrosion inhibitors are often added to commercial cleansers of which it is a component. It is possible that the amino group could act as a ligand under certain circumstances, as does the chloride ion for Fe-III, when hydrochloric acid is used in rust removal.
Sulfamic acid is used in the S.C. Johnson & Sons, Inc. "Scrubbing Bubbles Fizz-Its Toilet Tablets."
- Catalyst for esterification process
- Dye and pigment manufacturing
- Herbicide
- Ingredient in Denture Tablets
- Coagulator for urea-formaldehyde resins
- Ingredient in fire extinguishing media. Sulfamic acid is the main raw material for Ammonium sulfamate which is a widely used herbicide and fire retardant material for household product.
- Pulp and paper industry as a chloride stabilizer
- Synthesis of nitrous oxide by reaction with nitric acid
- In household cleaning chemical products such as Cameo.
- The deprotonated form (sulfamate) is a common counterion for Nickel(II) in electroplating.
References
This article has an unclear citation style. (September 2007) |
- ^ J. W. Bats, P. Coppens, T. F. Koetzle "The Experimental Charge Density in Sulfur-Containing Molecules: A Study of the Deformation Electron Density in Sulfamic Acid at 78 K by X-ray and Neutron Diffraction" Acta Crystallographica 1977, B33, pages 37–45.
- ^ a b R. L. Sass (1960). "A neutron diffraction study on the crystal structure of sulfamic acid". Acta Cryst. 13 (4): 320–324. doi:10.1107/S0365110X60000789.
{{cite journal}}: Unknown parameter|month=ignored (help)
- MSDS http://www.osha.gov [1]
- Sulfamates and their therapeutic potential. Med Res Rev. 2005 Mar;25(2):186-228. Review.
- R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0-471-95512-4
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.



