Sulfenic acid
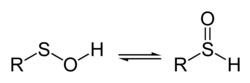
A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH, where R ≠ H. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively.
Properties
In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy (rotational spectroscopy) to be CH3–S–O–H.[1] Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form thiosulfinates, RS(O)SR, such as allicin from garlic. Through the use of X-ray crystallography, the structure of such stabilized sulfenic acids were shown to be R–S–O–H.[2][3] The stable, sterically hindered sulfenic acid, 1-triptycenesulfenic acid, has been found to have a pKa of 12.5 and an O–H bond-dissociation energy (bde) of 71.9 ± 0.3 kcal/mol, which can be compared to a pKa of ≥14 and O–H BDE of ∼88 kcal/mol for the (valence) isoelectronic hydroperoxides, ROOH.[4]
Formation and occurrence
Sulfenic acids are produced by the enzymatic decomposition of alliin and related compounds following tissue damage to garlic, onions, and other plants of the Allium genus. 1-Propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, the lachrymatory factor synthase, giving syn-propanethial-S-oxide.[5] 2-Propenesulfenic acid, formed from allicin, is thought to be responsible for garlic’s potent antioxidant activity.[6] Mass spectrometry with a DART ion source were used to identify 2-propenesulfenic formed when garlic is cut or crushed and to demonstrate that this sulfenic acid has a lifetime of less than one second.[7] The pharmacological activity of certain drugs, such as omeprazole, esomeprazole, ticlopidine, clopidogrel, and prasugrel is proposed to involve sulfenic acid intermediates.[8] Oxidation of cysteine residues in protein to the corresponding protein sulfenic acids is suggested to be important in redox-mediated signal transduction.[9]
Sulfenyl group
The prefix sulfenyl in organic nomenclature denotes the RS group (R≠H), for instance an arylsulfenylpyridine Ar-S-Py.[10]
References
- ^ Penn RE, Block E, Revelle LK (1978). "Methanesulfenic Acid". Journal of the American Chemical Society. 100 (11): 3622–3624. doi:10.1021/ja00479a068.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Goto K, Holler M, Okazaki R (1997). "Synthesis, Structure, and Reactions of a Sulfenic Acid Bearing a Novel Bowl-Type Substituent: The First Synthesis of a Stable Sulfenic Acid by Direct Oxidation of a Thiol". Journal of the American Chemical Society. 119 (6): 1460–1461. doi:10.1021/ja962994s.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Ishii A, Komiya K, Nakayama J (1996). "Synthesis of a Stable Sulfenic Acid by Oxidation of a Sterically Hindered Thiol (Thiophenetriptycene-8-thiol)1 and Its Characterization". Journal of the American Chemical Society. 118 (50): 12836–12837. doi:10.1021/ja962995k.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ McGrath AJ, Garrett GE, Valgimigli L, Pratt DA (2010). "The redox chemistry of sulfenic acids". Journal of the American Chemical Society. 132 (47): 16759–16761. doi:10.1021/ja1083046.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Block, E. (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 0-85404-190-7.
- ^ Vaidya V, Ingold KU, Pratt DA (2009). "Garlic: Source of the Ultimate Antioxidants – Sulfenic Acids". Angewandte Chemie. 121 (1): 163–6. doi:10.1002/ange.200804560. PMID 19040240.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Block E, Dane AJ, Thomas S, Cody RB (2010). "Applications of Direct Analysis in Real Time–Mass Spectrometry (DART-MS) in Allium Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane S-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums". Journal of Agricultural and Food Chemistry. 58 (8): 4617–4625. doi:10.1021/jf1000106. PMID 20225897.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: multiple names: authors list (link) - ^ Mansuy D, Dansette PM (2011). "Sulfenic acids as reactive intermediates in xenobiotic metabolism". Archives of Biochemistry and Biophysics. 507 (1): 174–185. doi:10.1016/j.abb.2010.09.015.
- ^ Kettenhofen, NJ, Wood, MJ (2010). "Formation, Reactivity, and Detection of Protein Sulfenic Acids". Chem. Res. Toxicol. 23 (11): 1633–1646. doi:10.1021/tx100237w.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ IUPAC Gold Book http://www.iupac.org/goldbook/S06098.pdf
