Triethyl citrate
Appearance
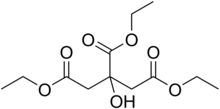
| |
| Names | |
|---|---|
| Preferred IUPAC name
Triethyl 2-hydroxypropane-1,2,3-tricarboxylate | |
| Other names
Ethyl citrate
E1505 Citric acid ethyl ester | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.974 |
| EC Number |
|
| E number | E1505 (additional chemicals) |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H20O7 | |
| Molar mass | 276.283 g/mol |
| Appearance | Oily liquid |
| Density | 1.137 g/mL at 25 °C |
| Melting point | −55 °C (−67 °F; 218 K)[2] |
| Boiling point | 294 °C (561 °F; 567 K) at 1 atm 235 °C at 150 mmHg |
| 65 g/L[2] | |
| -161.9·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triethyl citrate is an ester of citric acid. It is a colorless, odorless liquid used as a food additive (E number E1505) to stabilize foams, especially as whipping aid for egg white.[3] It is also used in pharmaceutical coatings and plastics.[4]
Triethyl citrate is also used as a plasticizer for polyvinyl chloride (PVC) and similar plastics.[5]
Triethyl citrate has been used as a pseudo-emulsifier in e-cigarette juices. It functions essentially like lecithin used in food products, but with the possibility of vaporization which lecithin does not have.
References
- ^ Triethyl citrate at Sigma-Aldrich
- ^ a b Record of Triethyl citrate in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ William J. Stadelman; Owen J. Cotterill (1995). Egg Science and Technology. Haworth Press. ISBN 1-56022-855-5.
- ^ Pharmaceutical Coatings Bulletin 102-4[permanent dead link], morflex.com
- ^ Hwan-Man Park; Manjusri Misra; Lawrence T. Drzal; Amar K. Mohanty (2004). ""Green" Nanocomposites from Cellulose Acetate Bioplastic and Clay: Effect of Eco-Friendly Triethyl Citrate Plasticizer". Biomacromolecules. 5 (6): 2281–2288. doi:10.1021/bm049690f. PMID 15530043.
{{cite journal}}: Unknown parameter|last-author-amp=ignored (|name-list-style=suggested) (help)
