Tropylium cation
In organic chemistry, the tropylium ion is an aromatic species with a formula of [C7H7]+.[1] Its name derives from the molecule tropine (itself named for the molecule atropine). Salts of the tropylium cation can be stable, e.g., tropylium tetrafluoroborate. It can be made from cycloheptatriene (tropylidene) and bromine or phosphorus pentachloride.[2]
It is a regular heptagonal, planar, cyclic ion; as well, it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms.
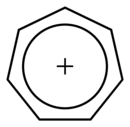 |
 |
 |
The structure shown is a composite of seven resonance contributors in which each carbon carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble salt from a reaction of cycloheptatriene and bromine.[3] The structure was elucidated by Eggers Doering and Knox in 1954.[4][5]
Mass spectrometry
The tropylium ion is frequently encountered in mass spectrometry in the form of a signal at m/z = 91 and is used in mass spectrum analysis. This fragment is often found for aromatic compounds containing a benzyl unit. Upon ionization, the benzyl fragment forms a cation (PhCH+
2), which rearranges to the highly stable tropylium cation (C
7H+
7).

7H+
8) and highest peak at m − 1 = 91 (C
7H+
7, stable tropylium cation).
Reactions
The tropylium cation reacts with nucleophiles to form substituted cycloheptatrienes, for example:[6]
- C
7H+
7 + CN−
→ C
7H
7CN
Reduction by lithium aluminum hydride yields cycloheptatriene.[6]
Reaction with a cyclopentadienide salt of sodium or lithium yields the covalently-bonded 7-cyclopentadienylcyclohepta-1,3,5-triene:[6]
- C
7H+
7X−
+ C
5H−
5Na+
→ C
7H
7C
5H
5 + NaX
When treated with oxidising agents such as chromic acid, the tropylium cation undergoes rearrangement into benzaldehyde:[6]
- C
7H+
7 + HCrO−
4 → C
6H
5CHO + CrO
2 + H
2O
See also
References
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molecule". doi:10.1351/goldbook.M04002
- ^ Tropylium tetrafluorate Organic Syntheses, Coll. Vol. 5, p.1138 (1973); Vol. 43, p.101 (1963). link
- ^ Merling, G. (1891). "Ueber Tropin". Berichte der deutschen chemischen Gesellschaft. 24: 3108–3126. doi:10.1002/cber.189102402151.
- ^ Eggers Doering, W. von; Knox, L. H. (1954). "The Cycloheptatrienylium (Tropylium) Ion". J. Am. Chem. Soc. 76 (12): 3203–3206. doi:10.1021/ja01641a027.
- ^ Balaban, Alexandru T.; Oniciu, Daniela C.; Katritzky, Alan R. (2004). "Aromaticity as a Cornerstone of Heterocyclic Chemistry". Chem. Rev. 104 (5): 2777–2812. doi:10.1021/cr0306790.
- ^ a b c d O. P. Agarwai (2009). Reactions and Reagents (46th ed.). Krishna Prakashan Media. pp. 614–615. ISBN 9788187224655.
