User:Ajc540/sandbox
The water-gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen (the mixture of carbon monoxide and hydrogen is known as water gas):
The water gas shift reaction was discovered by Italian physicist Felice Fontana in 1780. It wasn’t until much later when the industrial value of this reaction was better realized. Prior to early 20th century, hydrogen was obtained by reacting steam under high pressure with iron to produce iron, iron oxide and hydrogen. With the development of industrial processes that required hydrogen, such as the Haber-Bosch ammonia synthesis, the demand for a cheaper and more efficient method of hydrogen production was needed.[1] As a resolution to this problem, the WGSR was combined with the gassification of coal to produce a pure hydrogen product.
The pioneering period of the WGSR occurred between 1977 and 1984 after the discovery of metal carbonyls to catalyze the WGSR.[2] With this discovery came a plethora of publications on the ability of various metal catalysts to be used in the WGSR. While the majority of the groundbreaking research in water gas shift catalysis was conducted during this period, research in this area continues to be active in the present day.[1] [3]
At present, the Hydrogen economy ideal is gaining popularity and is becoming an important concept in both the political and research realms. Focus on hydrogen as a replacement fuel source for hydrocarbons is increasing. With this increasing interest, the WGSR has been receiving much attention over recent years. Considerable work has been done on developing new catalysts that will allow for a reduction in size and cost of WGSR for use in fuel cells.
Applications
[edit]Industrial
[edit]The WGSR is an important industrial reaction that is used in the manufacture of ammonia, hydrocarbons, methanol, and hydrogen. It is also often used in conjunction with steam reformation of methane and other hydrocarbons. In the Fischer-Tropsch process, the WGSR is one of the most important reactions used to balance the H2/CO ratio. It provides a source of hydrogen at the expense of carbon monoxide, which is important for the production of high purity hydrogen for use in ammonia synthesis. The water-gas shift reaction may be an undesired side reaction in processes involving water and carbon monoxide, e.g. the rhodium-based Monsanto process. The iridium-based Cativa process uses less water, which suppresses this reaction.
The equilibrium of this reaction shows a significant temperature dependence and the equilibrium constant decreases with an increase in temperature, that is, higher carbon monoxide conversion is observed at lower temperatures. In order to take advantage of both the thermodynamics and kinetics of the reaction, the industrial scale water gas shift reaction is conducted in multiple adiabatic stages consisting of a high temperature shift (HTS) followed by a low temperature shift (LTS) with intersystem cooling.[4] The initial HTS takes advantage of the high reaction rates, but is thermodynamically limited, which results in incomplete conversion of carbon monoxide and a 2-4% carbon monoxide exit composition. To shift the equilibrium toward hydrogen production, a subsequent low temperature shift reactor is employed to produce a carbon monoxide exit composition of less than 1%. The transition from the HTS to the LTS reactors necessitates intersystem colling. Due to the different reaction conditions, different catalysts must be employed at each stage to ensure optimal activity. The commercial HTS catalyst is the iron oxide–chromium oxide catalyst and the LTS catalyst is a copper-based catalyst. The order proceeds from high to low temperature due to the susceptibility of the copper catalyst to poisoning by sulfur that may remain after the steam reformation process.[5] This necessitates the removal of the sulfur compounds prior to the LTS reactor by a guard bed in order to protect the copper catalyst. Conversely, the iron used in the HTS reaction is generally more robust and resistant toward poisoning by sulfur compounds. While both the HTS and LTS catalysts are commercially available, their specific composition varies based on vendor. An important limitation for the HTS is the H2O/CO ratio where low ratios may lead to side reactions such as the formation of metallic iron, methanation, carbon deposition, and Fischer-Tropsch reaction.
High Temperature Shift Catalysts
[edit]The typical composition of commercial HTS catalyst has been reported as 74.2% Fe2O3, 10.0% Cr2O3, 0.2% MgO (remaining percentage attributed to volatile components).[6] The chromium acts to stabilize the iron oxide and prevents sintering. The operation of HTS catalysts occurs within the temperature range of 310 oC to 450 oC. The temperature increases along the length of the reactor due to the exothermic nature of the reaction. As such, the inlet temperature is maintained at 350 oC to prevent the exit temperature from exceeding 550 oC. Industrial reactors operate at a range from atmospheric pressure to 8375 kPa.[6]
Low Temperature Shift Catalysts
[edit]The typical composition of commercial LTS catalyst has been reported as 32-33% CuO, 34-53% ZnO, 15-33% Al2O3.[5] The active catalytic species is CuO. The function of ZnO is to provide structural support as well as prevent the poisoning of copper by sulfur. The Al2O3 prevents dispersion and pellet shrinkage. The LTS shift reactor operates at a range of 200 oC to 250 oC. Low reaction temperatures must be maintained due to the susceptibility of copper to thermal sintering. These lower temperatures also reduce the occurrence of side reactions that are observed in the case of the HTS.
Fuel cells
[edit]The WGSR can aid in the efficiency of fuel cells by increasing hydrogen production. The WGSR is considered a critical component in the reduction of carbon monoxide concentrations in cells that are susceptible to carbon monoxide poisoning such as the proton exchange membrane (PEM) fuel cell.[1] The benefits of this application are two-fold: not only would the water gas shift reaction effectively reduce the concentration of carbon monoxide, but it would also increase the efficiency of the fuel cells by increasing hydrogen production.[1] Unfortunately, current commercial catalysts that are used in industrial water gas shift processes are not compatible with fuel cell applications.[5] With the high demand for clean fuel and the critical role of the water gas shift reaction ion hydrogen fuel cells, the development of water gas shift catalysts for the application in fuel cell technology is an area of current research interest.
Catalysts
[edit]Catalysts for fuel cell application would need to be operable at low temperatures. Since the WGSR is kinetically slow at lower temperatures where equilibrium favors hydrogen production, WGS reactors require large amounts of catalysts, which increases their cost and size beyond practical application.[1] The commercial LTS catalyst used in large scale industrial plants is also pyrophoric in its inactive state and therefore presents significant safety concerns for consumer applications. [5] Interest in developing a catalyst that can overcome the current reaction rate limitations has increased along with increasing interest in a hydrogen economy.
Reaction Conditions
[edit]While the WGSR has been extensively studied for over a hundred years, there is still much debate regarding the mechanism of the reaction. A universal rate expression and mechanistic understanding have proven to be unattainabled due to the numerous amount of variables involved such as catalyst composition, active surface and structure of the catalyst, age of the catalyst, operating pressure and temperature, and gas composition.[4] These limitations are only further enhanced by the fact that commercial catalyst manufacturers rarely provide information about the catalysts.[4] This has resulted in numerous studies each with their individual rate expressions that are supported experimentally.[4]
Temperature Dependence
[edit]The water gas shift reaction is a moderately exothermic reversible reaction. Therefore with increasing temperature the reaction rate increases but the conversion of reactants to products becomes less favorable.[7] Due to its exothermic nature, high carbon monoxide conversion is thermodynamically favored at low temperatures. Despite the thermodynamic favorability at low temperatures, the reaction is kinetically favored at high temperatures. The water-gas shift reaction is sensitive to temperature, with the tendency to shift towards reactants as temperature increases due to Le Chatelier's principle. Over the temperature range 600 – 2000 K, the logarithm of the equilibrium constant for the WGSR is given by the following equation:[5]
The value of Keq approaches 1 at 1100 K. The following plot depicts the temperature dependence of Keq as shown by this equation.[5]

Mechanism
[edit]Two main mechanisms have have been proposed: an associative ‘Langmuir–Hinshelwood’ mechanism, and a regenerative ‘redox’ mechanism. While the regenerative mechanism is generally implemented to describe the WGS at higher temperatures, at low temperature both the redox and associative mechanisms are suitable explanations[8] .
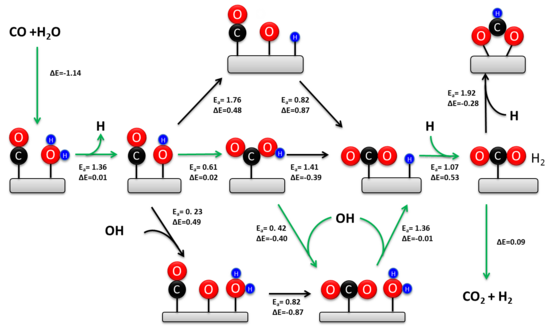
Associative Mechanism
[edit]In 1920 Armstrong and Hilditch first proposed the associative mechanism. In this mechanism CO and H2O are adsorbed onto the surface of the metal catalyst followed by the formation of an intermediate and the desorption of H2 and CO2. In the initial step, H2O dissociates into a metal adsorbed OH and H. The hydroxide then reacts with CO to form a carboxyl or formate intermediate which subsequently decomposes into CO2 and the metal adsorbed H, which ultimately yields H2. While this mechanism may be valid under LTS conditions, the redox mechanism which does not involve any long lived surface intermediates is a more suitable explanation of the WGS mechanism at higher temperatures.
Redox Mechanism
[edit]The regenerative ‘redox’ mechanism is the most commonly accepted mechanism for the WGSR. It involves a regenerative change in the oxidation state of the catalytic metal. In this mechanism, H2O is activated first by the abstraction of H from water followed by dissociation or disproportionation of the resulting OH to afford atomic O. The CO is then oxidized by the atomic O forming CO2 which returns the catalytic surface back to its pre-reaction state. Alternatively, CO may be directly oxidized by the OH to form a carboxyl intermediate, followed by the dissociation or disproportionation of the carboxyl. Finally H is recombined to H2 and CO2 and H2 are desorbed from the metal. The principal difference in these mechanisms is the formation of CO2. The redox mechanism generates CO2 by reaction with adsorbed oxygen, while the associative mechanism forms CO2 via the dissociation of an intermediate. The mechanism of decarboxylation is debated; it may involve β-hydride elimination, or it may require the action of an external base.
Reverse Water Gas Shift
[edit]
Depending on the reaction conditions, the equilibrium for the water gas shift can be pushed in either the forward or reverse direction. The reversibility of the WGSR is important in the production of ammonium, methanol, and Fischer-Tropsch synthesis where the ratio of H2/CO is critical. Many other industrial companies exploit the reverse water gas shift reaction (RWGS) reaction as a source of the synthetically valuable CO from cheap CO2. Typically, It is done using a copper on aluminium catalyst. The RWGS reaction is also gaining interest in the context of the human missions to Mars primarily for its potential to produce water and oxygen[9] [10] . The Mars atmosphere is about 95% CO2 which can be utilized by the RWGS reaction given a source of hydrogen. Coupling the RWGS with the water electrolysis process will yield methane and oxygen. Post electrolysis, the hydrogen produced can be recycled back into the RWGS reactor for the continued conversion of CO2. Because this reaction is only mildly endothermic, the thermal power needed to drive this reaction can potentially be produced by a Sabatier reactor.
See also
[edit]References
[edit]- ^ a b c d e Lamm, editors, Wolf Vielstich, Hubert Gasteiger, Arnold (2003). Handbook of fuel cells : fundamentals, technology, applications (Reprinted ed.). New York: Wiley. ISBN 0-471-49926-9.
{{cite book}}:|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Jacobs, G.; Davis, B. H. (2007). "Low temperature water-gas shift catalysts". Catalysis. 20: 122–285. doi:10.1039/B601305H. ISBN 978-0-85404-244-9.
{{cite journal}}: CS1 maint: date and year (link) - ^ Barakat, Tarek; Rooke, Joanna C.; Genty, Eric; Cousin, Renaud; Siffert, Stéphane; Su, Bao-Lian (1 January 2013). "Gold catalysts in environmental remediation and water-gas shift technologies". Energy & Environmental Science. 6 (2): 371–391. doi:10.1039/c2ee22859a.
- ^ a b c d Smith R J, Byron (2010). "A Review of the Water Gas Shift Reaction". International Journal of Chemical Reactor Engineering. 8: 1–32. doi:10.2202/1542-6580.2238. S2CID 96769998.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f Callaghan, Caitlin (2006). "Kinetics and Catalysis of the Water-Gas-Shift Reaction".
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b Newsome, David S. (1980). "The Water-Gas Shift Reaction". Catalysis Reviews: Science and Engineering. 21 (2): 275–318. doi:10.1080/03602458008067535.
- ^ Ratnasamy, Chandra; Wagner, Jon P. (2009). "Water Gas Shift Catalysis". Catalysis Reviews. 51 (3): 325–440. doi:10.1080/01614940903048661. S2CID 98530242.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ Guiseppe, Brenna (2010). "NEW CATALYST FOR THE H2 PRODUCTION BY WATER-GAS SHIFT REACTION PROCESSES". Dissertation.
- ^ Whitlow, Jonathan E. (2003). "Operation, Modeling and Analysis of the Reverse Water Gas Shift Process". AIP Conference Proceedings. Vol. 654. pp. 1116–1123. doi:10.1063/1.1541409. hdl:2060/20020050609.
{{cite book}}: CS1 maint: date and year (link) - ^ Zubrin, R. (3 November 1997). The Case for Mars. Free Press. p. 153. ISBN 0-684-83550-9.
Category:Inorganic reactions
Category:Chemical engineering
Category:Hydrogen production



