User:Mr. Ibrahem/Atovaquone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Mepron |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693003 |
| Routes of administration | By mouth |
| Drug class | Antiprotozoal[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 2.2–3.2 days |
| Identifiers | |
| |
| Chemical and physical data | |
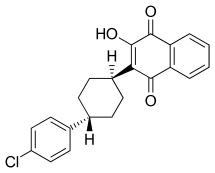
| Formula | C22H19ClO3 |
| Molar mass | 366.84 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 216 to 219 °C (421 to 426 °F) |
| |
| |
| | |
Atovaquone, sold under the brand name Mepron, is an medication used to treat and prevent Pneumocystis jirovecii pneumonia (PCP), toxoplasmosis and babesiosis.[3][1] For PCP it is used in those who cannot take trimethoprim/sulfamethoxazole.[3] It is taken by mouth.[3]
Common side effects include headache, fever, anxiety, trouble sleeping, vivid dreams, nausea, diarrhea, skin rash, and itching.[4] Other side effects may include liver problems and angioedema.[3] Safety in pregnancy and breastfeeding is unclear.[5] It is a quinone, specifically a naphthoquinone.[4][3]
Atovaquone was approved for medical use in the United States in 1992.[4] It is available as a generic medication.[6] In the United Kingdom 50 doses of 750 mg costs the NHS about £470 as of 2021.[6] This amount in the United States is about 220 USD.[7]
References[edit]
- ^ a b "Atovaquone Monograph for Professionals". Drugs.com. Archived from the original on 3 June 2021. Retrieved 16 January 2022.
- ^ "Wellvone 750mg/5ml oral suspension - Summary of Product Characteristics (SmPC)". (emc). 28 November 2019. Archived from the original on 24 October 2020. Retrieved 18 September 2020.
- ^ a b c d e f "Atovaquone Oral SUSPENSION- atovaquone suspension". DailyMed. 10 December 2019. Archived from the original on 7 August 2020. Retrieved 18 September 2020.
- ^ a b c d "Atovaquone". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 16 January 2022.
- ^ "Atovaquone (Mepron) Use During Pregnancy". Drugs.com. Archived from the original on 28 November 2020. Retrieved 16 January 2022.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 641. ISBN 978-0857114105.
- ^ "Atovaquone Prices and Atovaquone Coupons - GoodRx". GoodRx. Retrieved 16 January 2022.
