User:Mr. Ibrahem/Paroxetine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Paxil, Seroxat, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Selective serotonin reuptake inhibitor (SSRI)[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extensively absorbed from the GI tract, but extensive first-pass metabolism in the liver[2][3][4][5] |
| Protein binding | 93–95%[2][3][4] |
| Metabolism | Extensive, hepatic (mostly CYP2D6-mediated)[2][3][4] |
| Elimination half-life | 21 hours[2][3][4] |
| Excretion | Kidney (64%; 2% unchanged and 62% as metabolites), faecal (36%; <1% unchanged)[2][3][4] |
| Identifiers | |
| |
| Chemical and physical data | |
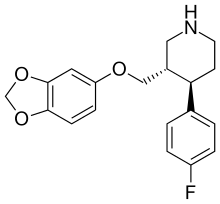
| Formula | C19H20FNO3 |
| Molar mass | 329.3 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Paroxetine, sold under the brand names Paxil and Seroxat among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[1] It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, posttraumatic stress disorder, generalized anxiety disorder and premenstrual dysphoric disorder.[1] It has also been used in the treatment of premature ejaculation and hot flashes due to menopause.[1][7] It is taken by mouth.[1]
Common side effects include drowsiness, dry mouth, loss of appetite, sweating, trouble sleeping, and sexual dysfunction.[1] Serious side effects may include suicide in those under the age of 25, serotonin syndrome, and mania.[1] While the rate of side effects appears similar compared to other SSRIs and SNRIs, antidepressant discontinuation syndromes may occur more often.[8][9] Use in pregnancy is not recommended while use during breastfeeding is relatively safe.[10] It is believed to work by blocking the re-uptake of the chemical serotonin by neurons in the brain.[1]
Paroxetine was approved for medical use in the United States in 1992 and initially sold by GlaxoSmithKline.[1][11] It is available as a generic medication.[12] A month supply in the United Kingdom costs the NHS about £1.10 per month as of 2019.[12] In the United States the wholesale cost of this amount is about US$2.40.[13] In 2017, it was the 68th most commonly prescribed medication in the United States, with more than eleven million prescriptions.[14][15] The United States Department of Justice fined GlaxoSmithKline $3 billion in 2012, for withholding data, unlawfully promoting use in those under 18, and preparing an article that misleadingly reported the effects of paroxetine in adolescent with depression following its clinical trial study 329.[16][17][18]
References[edit]
- ^ a b c d e f g h i j k "Paroxetine Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 6 March 2019. Retrieved 3 March 2019.
- ^ a b c d e Sandoz Pty Ltd (18 January 2012). "PRODUCT INFORMATION PAROXETINE SANDOZ 20mg FILM-COATED TABLETS" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. Archived from the original on 4 September 2015. Retrieved 22 November 2013.
- ^ a b c d e Mylan Institutional Inc. (January 2012). "PAROXETINE (paroxetine hydrochloride hemihydrate) tablet, film coated". DailyMed. U.S. National Library of Medicine. Archived from the original on 23 October 2013. Retrieved 22 November 2013.
- ^ a b c d e Sandoz Limited (21 March 2013). "Paroxetine 20 mg Tablets – Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Datapharm Ltd. Archived from the original on 3 December 2013. Retrieved 22 November 2013.
- ^ "Paxil, Paxil CR (paroxetine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 10 November 2015. Retrieved 22 November 2013.
- ^ "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 7 September 2020.
- ^ Fischer, Andrea (June 28, 2013). "FDA approves the first non-hormonal treatment for hot flashes associated with menopause" (Press release). Food and Drug Administration. Archived from the original on January 18, 2017.
- ^ Hosenbocus S, Chahal R (February 2011). "SSRIs and SNRIs: A review of the Discontinuation Syndrome in Children and Adolescents". Journal of the Canadian Academy of Child and Adolescent Psychiatry. 20 (1): 60–7. PMC 3024727. PMID 21286371.
- ^ Pae CU, Patkar AA (February 2007). "Paroxetine: current status in psychiatry". Expert Review of Neurotherapeutics. 7 (2): 107–20. doi:10.1586/14737175.7.2.107. PMID 17286545. S2CID 34636522.
- ^ "Paroxetine Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 3 December 2018. Retrieved 3 March 2019.
- ^ Food and Drug Administration (2011). Approved Drug Products with Therapeutic Equivalence Evaluations – FDA Orange Book 31st Edition (2011): FDA Orange Book 31st Edition (2011). DrugPatentWatch.com. p. 344. ISBN 9781934899816. Archived from the original on 2019-03-06. Retrieved 2019-03-04.
- ^ a b British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 363. ISBN 9780857113382.
- ^ "NADAC as of 2019-02-27". Centers for Medicare and Medicaid Services. Retrieved 3 March 2019.
- ^ "The Top 300 of 2020". ClinCalc. Archived from the original on 2020-03-18. Retrieved 11 April 2020.
- ^ "Paroxetine - Drug Usage Statistics". ClinCalc. Archived from the original on 11 April 2020. Retrieved 11 April 2020.
- ^ "GlaxoSmithKline to Plead Guilty and Pay $3 Billion to Resolve Fraud Allegations and Failure to Report Safety Data" (Press release). United States Department of Justice, Office of Public Affairs. 2 July 2012. Archived from the original on 9 September 2014. Retrieved 6 October 2015.
The United States alleges that, among other things, GSK participated in preparing, publishing and distributing a misleading medical journal article that misreported that a clinical trial of Paxil demonstrated efficacy in the treatment of depression in patients under age 18, when the study failed to demonstrate efficacy.
- ^ United States ex rel. Greg Thorpe, et al. v. GlaxoSmithKline PLC, and GlaxoSmithKline LLC, pp. 3–19 (D. Mass. 26 October 2011), Text.
- ^ Thomas, Katie; Schmidt, Michael S. (2 July 2012). "Glaxo Agrees to Pay $3 Billion in Fraud Settlement". The New York Times. Archived from the original on 2 March 2017. Retrieved 28 February 2017.
