User:Shalso/sandbox
About this draft sandbox page
[edit]The following is a Draft of modifications to the existing Removal Processes section on the Atmospheric methane page. Section in RED are the ISA related edits/additions.
Removal processes
[edit]Any process that consumes methane from the atmosphere can be considered a "sink" of atmospheric methane. The most prominent of these processes occur as a result of methane either being destroyed in the atmosphere or broken down in soil. Humans have not acted as a significant sink of atmospheric methane, although the Iron Salt Aerosol method described below could be used as an artificial method of methane removal from the atmosphere which mimics the natural removal process caused by naturally occurring iron rich atmospheric dust[1].
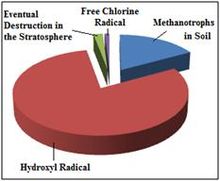
Reaction with the hydroxyl radical – The major removal mechanism of methane from the atmosphere involves radical chemistry; it reacts with the hydroxyl radical (·OH) in the troposphere or stratosphere to create the ·CH3 radical and water vapor. In addition to being the largest known sink for atmospheric methane, this reaction is one of the most important sources of water vapor in the upper atmosphere. Following the reaction of methane with the hydroxyl radical, two dominant pathways of methane oxidation exist: [1] which leads to a net production of ozone, and [2] which causes no net ozone change. For methane oxidation to take the pathway that leads to net ozone production, nitric oxide (NO) must be available to react with CH3O2·. Otherwise, CH3O2· reacts with the hydroperoxyl radical (HO2·), and the oxidation takes the pathway with no net ozone change. Both oxidation pathways lead to a net production of formaldehyde and water vapor.
[1] Net production of O3
CH4 + ·OH → CH3· + H2O
CH3· + O2 + M → CH3O2· + M
CH3O2· + NO → NO2 + CH3O·
CH3O· + O2 → HO2· + HCHO
HO2· + NO → NO2 + ·OH
(2x) NO2 + hv → O(3P) + NO
(2x) O(3P) + O2 + M → O3 + M
[NET: CH4 + 4O2 → HCHO + 2O3 + H2O]
[2] No net change of O3
CH4 + ·OH → CH3· + H2O
CH3· + O2 + M → CH3O2· + M
CH3O2· + HO2· + M → CH3O2H + O2 + M
CH3O2H + hv → CH3O· + ·OH
CH3O· + O2 → HO2· + HCHO
[NET: CH4 + O2 → HCHO + H2O]
Note that for the second reaction, there will be a net loss of radicals in the case where CH3O2H is lost to wet deposition before it can undergo photolysis such that: CH3O2H + H2O → wet deposition. Also note that M represents a random molecule that facilitates energy transfer during the reaction[2]
This reaction in the troposphere gives a methane lifetime of 9.6 years. Two more minor sinks are soil sinks (160 year lifetime) and stratospheric loss by reaction with ·OH, ·Cl and ·O1D in the stratosphere (120 year lifetime), giving a net lifetime of 8.4 years.[3] Oxidation of methane is the main source of water vapor in the upper stratosphere (beginning at pressure levels around 10 kPa).
The methyl radical formed in the above reaction will, during normal daytime conditions in the troposphere, usually react with another hydroxyl radical to form formaldehyde. Note that this is not strictly oxidative pyrolysis as described previously. Formaldehyde can react again with a hydroxyl radical to form carbon dioxide and more water vapor. Sidechains in these reactions may interact with nitrogen compounds that will likely produce ozone, thus supplanting radicals required in the initial reaction.[4]
Natural sinks of atmospheric methane
[edit]Most natural sinks occur as a result of chemical reactions in the atmosphere as well as oxidation by methane consuming bacteria in Earth’s soils.
Methanotrophs in soils
[edit]Soils act as a major sink for atmospheric methane through the methanotrophic bacteria that reside within them. This occurs with two different types of bacteria. "High capacity-low affinity" methanotrophic bacteria grow in areas of high methane concentration, such as waterlogged soils in wetlands and other moist environments. And in areas of low methane concentration, "low capacity-high affinity" methanotrophic bacteria make use of the methane in the atmosphere to grow, rather than relying on methane in their immediate environment.[5]
Forest soils act as good sinks for atmospheric methane because soils are optimally moist for methanotroph activity, and the movement of gases between soil and atmosphere (soil diffusivity) is high.[5] With a lower water table, any methane in the soil has to make it past the methanotrophic bacteria before it can reach the atmosphere.
Wetland soils, however, are often sources of atmospheric methane rather than sinks because the water table is much higher, and the methane can be diffused fairly easily into the air without having to compete with the soil’s methanotrophs.
Methanotrophic bacteria in soils – Methanotrophic bacteria that reside within soil use methane as a source of carbon in methane oxidation.[5] Methane oxidation allows methanotrophic bacteria to use methane as a source of energy, reacting methane with oxygen and as a result producing carbon dioxide and water.
- CH4 + 2O2 → CO2 + 2H2O
Troposphere
[edit]The most effective sink of atmospheric methane is the hydroxyl radical in the troposphere, or the lowest portion of Earth’s atmosphere. As methane rises into the air, it reacts with the hydroxyl radical to create water vapor and carbon dioxide. The lifespan of methane in the atmosphere was estimated at 9.6 years as of 2001; however, increasing emissions of methane over time reduce the concentration of the hydroxyl radical in the atmosphere.[6] With less OH˚ to react with, the lifespan of methane could also increase, resulting in greater concentrations of atmospheric methane.[7]
Stratosphere
[edit]If it is not destroyed in the troposphere, methane will last approximately 120 years before it is eventually destroyed in Earth’s next atmospheric layer: the stratosphere. Destruction in the stratosphere occurs the same way that it does in the troposphere: methane is oxidized to produce carbon dioxide and water vapor. Based on balloon-borne measurements since 1978, the abundance of stratospheric methane has increased by 13.4%±3.6% between 1978 and 2003.[8]
Reaction with free chlorine
[edit]The reaction of methane and chlorine atoms acts as a primary sink of Cl atoms and is a primary source of hydrochloric acid (HCl) in the stratosphere.[2]
CH4 + Cl → CH3 + HCl
The HCl produced in this reaction leads to catalytic ozone destruction in the stratosphere.[9]
Proposed artificial sinks of atmospheric methane
[edit]Artificial methane removal may prevent dangerous self perpetuating runaway climate change scenarios emerging which some currently see as inevitable[10].
There is currently one peer reviewed scientific proposal involving artificial removal of methane from the atmosphere: the Iron Salt Aerosol method[1].
Iron Salt Aerosol
[edit]This climate engineering technique proposes adding Iron Salt Aerosol to the troposphere.
Question for Robert/Franz: What studies have been done that can be quoted here into the natural process of methane removal by naturally occurring iron rich dust? This could be used as the starting point for this section in order to make it obvious it is an already occurring process in nature.
This would, in the presence of sunshine and naturally occurring sea-salt-generated hydrogen chloride, emit chlorine (°Cl) atoms (free radicals) into the troposphere.
Chlorine atoms are a powerful oxidising agent, and in enhanced quantity remove tropospheric methane and ozone up to 250 times faster (depending on humidity) than oxidation by Hydroxyl radical (°OH) radicals. The exact process is described here:
Iron(III) chlorides (ISA) are photolyzed by daylight to Iron(II) chlorides and chlorine (°Cl) atoms. The °Cl atoms react with methane (CH4) in the following way:-
CH4 + Cl → CH3 + HCl
The HCl produced in this reaction will oxidise ozone in the low level troposphere, where ozone acts as a powerful greenhouse gas, but will not affect protective stratospheric ozone.
Question for Franz: What happens to the CH3? How does methane + chlorine end up as carbon dioxide?
- ^ a b Franz Dietrich Oeste; Renaud de Richter; Tingzhen Ming; Sylvain Caillol (13 January 2017). "Climate engineering by mimicking natural dust climate control: the iron salt aerosol method". Earth System Dynamics. 8 (1): 1–54. doi:10.5194/esd-8-1-2017.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Warneck, Peter (2000). Chemistry of the Natural Atmosphere. Academic Press. ISBN 9780127356327.
- ^ Cite error: The named reference
Trace Gaseswas invoked but never defined (see the help page). - ^ Loïc Jounot (2006). "Tropospheric Chemistry". University of Toronto Atmospheric Physics Department. Archived from the original on 17 June 2008. Retrieved 2008-07-18.
- ^ a b c Reay, Dave. "Methane Sinks − Soils". Greenhouse Gas Online. Retrieved 2016-12-22.
- ^ Cite error: The named reference
EPAwas invoked but never defined (see the help page). - ^ Holmes, C. D.; et al. (January 2013). "Future methane, hydroxyl, and their uncertainties: key climate and emission parameters for future predictions". Atmospheric Chemistry and Physics. 13 (1): 285–302. Bibcode:2013ACP....13..285H. doi:10.5194/acp-13-285-2013.
{{cite journal}}: CS1 maint: unflagged free DOI (link) See Table 2. - ^ Rohs, S.; Schiller, C.; Riese, M.; Engel, A.; Schmidt, U.; Wetter, T.; Levin, I.; Nakazawa, T. (July 2006). "Long-term changes of methane and hydrogen in the stratosphere in the period 1978-2003 and their impact on the abundance of stratospheric water vapor". Journal of Geophysical Research: Atmospheres. 111 (D14): D14315. Bibcode:2006JGRD..11114315R. doi:10.1029/2005JD006877. D14315.
- ^ Rohs, S.; Schiller, C.; Riese, M.; Engel, A.; Schmidt, U.; Wetter, T.; Levin, I.; Nakazawa, T.; Aoki, S. (2006). "Long-term changes of methane and hydrogen in the stratosphere in the period 1978–2003 and their impact on the abundance of stratospheric water vapor". Journal of Geophysical Research. 111 (D14). doi:10.1029/2005jd006877. ISSN 0148-0227.
- ^ Gail Whiteman; Chris Hope; Peter Wadhams (July 25, 2013). "Vast costs of Arctic change". Nature. Retrieved April 20, 2019.

