User:SushiLover135/sandbox
Peer review
[edit]General info
[edit]- Whose work are you reviewing? KeyLowZip
- Link to draft you're reviewing: User:KeyLowZip/Transferrin/SushiLover135 Peer Review
Plastocyanin
[edit]
1st 250 word contribution
[edit]Function
[edit]More specifically, Plastocyanin has three main functions. Since the electron transfer reaction between cytochrome f and P007+ is a reduction-oxidation (redox) reaction, plastocyanin maintains the redox potential between the two. The second function is that it must have the copper center to act as the catalyst in the redox reaction. The last main function of plastocyanin is to provide an adequate structure to allow for electrons to transfer with relative ease.[1]
Structure
[edit]Plastocyanin is a derivative of a Blue-Copper Protein. The reason why this is a blue-copper protein is because of its spectroscopic characteristics and the parameters that it can be analyzed in. Methods that have been used to characterize the structure of the Plastocyanin protein includes X-ray Crystallography and Nuclear Magnetic Resonance (NMR) Spectrometry. The poplar plastocyanin structure was characterized 1978 using such methods. In 1978, samples which contained plastocyanin proteins, such as algae, parsley and French bean plants were used to determine plastocyanin’s structure.[2]
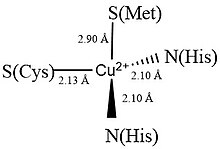
The structure of plastocyanin is an eight-stranded antiparallel β-barrel that contains one copper metal center. The ligands coordinated to the copper center are two Histidines (His37 & His87), a Cysteine (Cys84) and a Methionine (Met92). [1][2]
This blue-copper protein is the catalyst to facilitate the electron transfer reaction from the cytochrome f in the b6f complex to P007+ in Photosystem I. The protein itself is comprised of 97-104 amino acids.[2]
Crystallization Method
[edit]
Spinach was a very common source of plastocyanin proteins. It was commonly used for experiments due to its availability and its low difficulty to extract/prepare the plastocyanin samples. The crystallization method involved am method known as mutagenesis. The crystals can then be grown using periplasmic preparations. This preparation is meant to allow X-ray Crystallography to be utilized in order to characterize the molecule. [2]
Entatic State
[edit]The first thing to understand is that a catalyst’s function is to increase the speed of the electron transfer (redox) reaction. Plastocyanin is believed to work less like an enzyme where enzymes decrease the transition energy needed to transfer the electron. Plastocyanin works more on the principles of entatic states where it increases the energy of the reactants, decreasing the amount of energy needed for the redox reaction to occur. Another was to rephrase the function of plastocyanin is that it can facilitate the electron transfer reaction by providing a small reorganization energy, which has been measured to about 16-28 kcal/mol. [3]
To study the properties of the redox reaction of plastocyanin, methods such as quantum mechanics / molecular mechanics (QM/MM) molecular dynamics simulations. This method was used to determine that Plastocyanin has an entatic strain energy of about 10 kcal/mol. [3]
Plastocyanin in the Ocean
[edit]Usually, plastocyanin can be found in organisms that contain chlorophyll b and cyanobacteria, as well as algae that contain chlorophyll c. Plastocyanin has also been found in the diatom, Thalassiosira oceanica, which can be found in oceanic environments. It was surprising to find these organisms containing the protein, plastocyanin, because the concentration of copper dissolved in the ocean is low (between 0.4 – 50 nM). However, when the concentration of copper in the oceans is compared to the concentrations of other metals such as Zinc and Iron, the concentration of copper is much higher. Other organisms that live in the ocean, such as phytoplankton, have adapted to where they don’t need these low concentration metals (Fe & Zn) to facilitate photosynthesis and grow.[4]
References
[edit]- ^ a b Redinbo, Matthew R.; Yeates, Todd O.; Merchant, Sabeeha (1994-02-01). "Plastocyanin: Structural and functional analysis". Journal of Bioenergetics and Biomembranes. 26 (1): 49–66. doi:10.1007/BF00763219. ISSN 1573-6881.
- ^ a b c d Xue, Yafeng; Ökvist, Mats; Hansson, Örjan; Young, Simon (1998). "Crystal structure of spinach plastocyanin at 1.7 Å resolution". Protein Science. 7 (10): 2099–2105. doi:10.1002/pro.5560071006. ISSN 1469-896X. PMC 2143848. PMID 9792096.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b Hurd, Catherine A.; Besley, Nicholas A.; Robinson, David (2017-06-15). "A QM/MM study of the nature of the entatic state in plastocyanin". Journal of Computational Chemistry. 38 (16): 1431–1437. doi:10.1002/jcc.24666. PMC 5434870. PMID 27859435.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Peers, Graham; Price, Neil M. (2006-5). "Copper-containing plastocyanin used for electron transport by an oceanic diatom". Nature. 441 (7091): 341–344. doi:10.1038/nature04630. ISSN 0028-0836.
{{cite journal}}: Check date values in:|date=(help)
