Zinc acetate

| |
| Names | |
|---|---|
| IUPAC name
Zinc acetate
| |
| Other names
Acetic acid, Zinc salt
Acetic acid, Zinc(II) salt Dicarbomethoxyzinc Zinc diacetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.338 |
| E number | E650 (flavour enhancer) |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O6Zn (dihydrate) | |
| Molar mass | 219.50 g/mol (dihydrate) 183.48 g/mol (anhydrous) |
| Appearance | White solid (all forms) |
| Density | 1.735 g/cm3 (dihydrate) |
| Melting point | Decomposes 237 °C (dihydrate loses water at 100 °C) |
| Boiling point | decomp. |
| 43 g/100 mL (20 °C, dihydrate) | |
| Solubility | soluble in alcohol |
| Structure | |
| octahedral (dihydrate) | |
| tetrahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
mildly toxic |
| Related compounds | |
Other anions
|
Zinc chloride |
Other cations
|
Copper(II) acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Zinc acetate is the chemical compound with the formula Zn(O2CCH3)2, which commonly occurs as a dihydrate Zn(O2CCH3)2(H2O)2. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on zinc carbonate or zinc metal. When used as a food additive, it has the E number E650.
Basic properties and structures
The acetate group is capable of binding to metal ions in a variety of ways through its two oxygen atoms and several connectivities are observed for the various hydrates of zinc acetate. Anhydrous zinc acetate adopts a polymeric structure consisting of zinc coordinated to four oxygen atoms in a tetrahedral environment, each tetrahedron being connected to neighbors by the acetate groups.[1] The acetate ligands are not bidentate. In contrast, most metal diacetates feature metals in octahedral coordination with bidentate acetate groups. In zinc acetate dihydrate the zinc is octahedral, wherein both acetate groups are bidentate.[2]
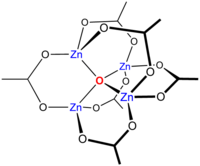
Basic zinc acetate
Heating Zn(CH3CO2)2 in a vacuum results in loss of acetic anhydride, leaving a residue of "basic zinc acetate," with the formula Zn4O(CH3CO2)6. This cluster compound has the tetrahedral structure shown below. This species closely resembles the corresponding beryllium compound, although it is slightly expanded with Zn-O distances ~1.97 vs ~1.63 Å for Be4O(OAc)6.[3]
Applications
Dietary and medicinal applications
Zinc acetate is used as a dietary supplement and in lozenges used to treat the common cold. Zinc acetate alone is thought to be a more effective treatment than zinc gluconate.[4]
Zinc acetate can also be used to treat zinc deficiencies. As an oral daily supplement it is used to inhibit the body's absorption of copper as part of the treatment for Wilson's disease. Zinc acetate is also sold as an astringent in the form of an ointment, a topical lotion; or combined with an antibiotic such as erythromycin for the topical treatment of acne. Furthermore Zinc acetate is commonly sold as a topical anti-itch ointment.
In chewing gum, zinc acetate is a breath freshener[5][6] and plaque inhibitor[7].
Industrial applications
Industrial applications include wood preserving, manufacturing other zinc salts, polymers, manufacture of ethylene acetate, as a dye mordant, and analytical reagent.
Zinc acetate is a precursor via a sol-gel route to the transparent semiconductor zinc oxide.
References
- ^ Capilla, A. V.; Aranda, R. A. (1979). "Anhydrous Zinc(II) Acetate (CH3-COO)2Zn". Crystal Structure Communications. 8: 795–797.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ J. N. van Niekerk, F. R. L. Schoening and J. H. Talbot (1953). "The crystal structure of zinc acetate dihydrate, Zn(CH3COO)2·2H2O". Acta Cryst. 6 (8): 720–723. doi:10.1107/S0365110X53002015.
- ^ Koyama, H.; Saito, Y. (1954). "The Crystal Structure of Zinc Oxyacetate, Zn4O(CH3COO)6". Bull. Chem. Soc. Japan. 27 (2): 112–114. doi:10.1246/bcsj.27.112.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eby GA (2004). "Zinc lozenges: cold cure or candy? Solution chemistry determinations" (PDF). Biosci. Rep. 24 (1): 23–39. doi:10.1023/B:BIRE.0000037754.71063.41. PMID 15499830.
- ^ US 7087255
- ^ US 6592849
- ^ Giertsen E, Svatun B, Saxton A (1987). "Plaque inhibition by hexetidine and zinc". Scand J Dent Res. 95 (1): 49–54. PMID 3470899.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)