Acetanilide
| Acetanilide | |
|---|---|
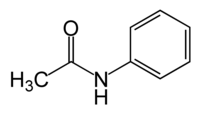
| |
| Chemical name | N-phenylacetamide |
| Chemical formula | C8H9NO |
| Molecular mass | 135.17 g/mol |
| CAS number | [103-84-4] |
| Density | 1.219 g/cm³ |
| Melting point | 113–115 °C (235–239 °F) |
| Boiling point | 304 °C |
| Solubility | 1g/185mL |
| Water solubility | 0.1g/100mL at 22 °C |
| SMILES | O=C(C)Nc1ccccc1 |
| Disclaimer and references | |
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name antifebrin.
Formation
Acetanilide can be produced by reacting acetic anhydride with either aniline or phenylammonium chloride
Properties
This compound is soluble in hot water. It has the ability to self-ignite if it reaches a temperature of 545 °C, but is otherwise stable under most conditions. Pure crystals are white in colour.
Applications
Acetanilide is used as an inhibitor in hydrogen peroxide and is used to stabilize cellulose ester varnishes. It has also found uses in the intermediation in rubber accelerator synthesis, dyes and dye intermediate synthesis, and camphor synthesis. Acetanilide was used as a precursor in penicillin synthesis and other pharmaceuticals and its intermediates.
Acetanilide has analgesic and fever-reducing properties; it is in the same class of drugs as acetaminophen (paracetamol). Under the name acetanilid it formerly figured in the formula of a number of patent medicines and over the counter drugs. In 1948, Julius Axelrod and Bernard Brodie discovered that acetanilide is much more toxic in these applications than other drugs, causing methemoglobinemia and ultimately doing damage to the liver and kidneys. As such, acetanilide has largely been replaced by less toxic drugs, in particular acetaminophen, which is a metabolite of acetanilide and whose use Axelrod and Brodie suggested in the same study.
In the 19th century it was one of a large number of compounds used as experimental photographic developers.
