β-Carboline
This article needs additional citations for verification. (December 2007) |
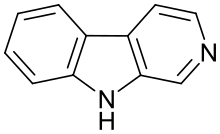
| |
| Names | |
|---|---|
| IUPAC name
9H-β-carboline
| |
| Other names
9H-pyrido[3,4-b]indole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.005.418 |
| MeSH | norharman |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H8N2 | |
| Molar mass | 168.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Carboline (9H-pyrido[3,4-b]indole) is an organic amine that is the prototype of a class of compounds known as β-carbolines.
Pharmacology
β-carboline alkaloids are widespread in plants and animals, and frequently act as monoamine oxidase inhibitors (MAOI). As components of the liana Banisteriopsis caapi, the β-carbolines harmine, harmaline, and tetrahydroharmine play a pivotal role in the pharmacology of the psychedelic brew ayahuasca. Some β-carbolines, notably tryptoline and pinoline, are formed naturally in the human body. The latter is implicated along with melatonin in the role of the pineal gland in regulating the sleep-wake cycle.[citation needed] The β-carboline can link to cerebral benzodiazepine receptors and induce inverse agonist effect.
United States Patent Number 5591738 describes a method for treating various chemical dependencies via the administration of beta-carbolines.[1]
Structure
The structure of β-carboline is similar to that of tryptamine, with the ethylamine chain re-connected to the indole ring via an extra carbon atom, to produce a three-ringed structure. Indeed, biosynthesis of β-carbolines is believed to follow this route from analogous tryptamines. Different levels of saturation are possible in the third ring, which is indicated here in the structural formula by colouring the optionally double bonds red and blue:
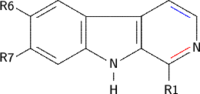
Examples of β-carbolines
Some of the more important β-carbolines are tabulated by structure below.
| Short Name | R1 | R6 | R7 | Structure | ||
|---|---|---|---|---|---|---|
| β-Carboline |  | |||||
| Tryptoline | 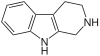 | |||||
| Pinoline |  | |||||
| Harmane |  | |||||
| Harmine | 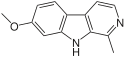 | |||||
| Harmaline |  | |||||
| Tetrahydroharmine |  |
Occurrence in nature
"There are presently 64 known β-carboline alkaloids dispersed throughout at least eight plant families."[1] The seeds of Peganum harmala (Syrian Rue) are a good source of beta-carbolines, since they contain about 2-6% alkaloids, most of which is harmaline.[2][unreliable source?]
As a result of the presence of Beta-carbolines in the cuticle of Scorpions, they are known to glow when exposed to certain wavelengths of ultraviolet light such as that produced by a blacklight.[3]
See also
External links
- TiHKAL #44
- TiHKAL in general
- Beta-Carbolines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Beta-carbolines in Coffee
- Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test
References
- ^ a b Method of treating chemical dependency using .beta.-carboline alkaloids, derivatives and salts thereof
- ^ www.amazing-nature.com
- ^ Stachel, Shawn J (1999). "The fluorescence of scorpions and cataractogenesis". Chemistry & Biology. 6. Cell Press: 531–539. doi:10.1016/S1074-5521(99)80085-4. Retrieved 2008-06-17.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)
