Cuttlebone
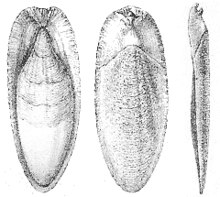




Cuttlebone, also known as cuttlefish bone, is a hard, brittle internal structure (an internal shell) found in all members of the family Sepiidae, commonly known as cuttlefish, within the cephalopods. In other cephalopod families it is called a gladius.
Cuttlebone is composed primarily of aragonite. It is a chambered structure that the animal can fill with gas or liquid for buoyancy control. On the ventral (bottom) side of the cuttlebone is the highly modified siphuncle; this is the organ with which the cuttlebone is filled with gas or liquid.[2] The microscopic structure of cuttlebone consists of narrow layers connected by numerous upright pillars.
Depending on the species, cuttlebones implode at a depth of 200 to 600 metres (660 to 1,970 ft). Because of this limitation, most species of cuttlefish live on the seafloor in shallow water, usually on a continental shelf.[3]
When the cuttlefish dies, only the cuttlebone remains and will often wash up on a beach.
Human uses
[edit]In the past, cuttlebones were ground up to make polishing powder, which was used by goldsmiths.[4] The powder was also added to toothpaste,[5] and was used as an antacid for medicinal purposes[4] or as an absorbent. They were also used as an artistic carving medium during the 19th[6][7] and 20th centuries.[8][9][10][11][12]
Today, cuttlebones are commonly used as calcium-rich dietary supplements for caged birds, chinchillas, hermit crabs, reptiles, shrimp, and snails. These are not intended for human consumption.[13][14]
Lime production
[edit]As a carbonate-rich biogenic raw material, cuttlebone has potential to be used in the production of calcitic lime.[15]
Jewelry making
[edit]Because cuttlebone is able to withstand high temperatures and is easily carved, it serves as mold-making material for small metal castings for the creation of jewelry and small sculptural objects.[a]
It can also be used in the process of pewter casting, as a mould.
Internal structure
[edit]The microstructure of the cuttlebone consists of two components, horizontal septa and vertical pillars. Both components are composed predominantly of aragonite.[16] The horizontal septa divide the cuttlebone into separate chambers. These chambers are supported by the vertical pillars which have a corrugated (or "wavy") structure.[16] The thickness of these pillars varies from species to species, but are typically a few microns thick.[16][17] The horizontal septa are typically thicker than the vertical pillars and consist of a double-layered structure. The upper layer of the septa and walls consist of vertically aligned crystals, whereas the bottom sublayer consists of nanorods rotated with respect to each other to form a "plywood" structure.[17] Overall, this chambered microstructure results in the cuttlebone having a porosity over 90% by volume.[17]
- 3D visualisation of a Sepia cuttlebone by industrial micro-computed tomography
-
3D view of part of a cuttlebone at low resolution.
-
Overview of a part at high resolution, about 5 μm/voxel.
-
Higher magnification.
-
Detailed view at very high magnification. Wall thickness of the vertical structures is about 10 μm.
- Flight through the corresponding tomographic image stacks
-
Flight through the corresponding μCT image stack, section direction about 30°, lateral view.
-
Flight through the corresponding μCT image stack, section direction about 30°, top view.
-
Flight through the aligned image stack, lateral view.
-
Flight through the aligned image stack, top view.
-
Flight through the aligned image stack, top view, magnified section.
Mechanical properties
[edit]| Part of a series related to |
| Biomineralization |
|---|
 |
The cuttlebone has been studied extensively due to its ability to be simultaneously lightweight, stiff, and tolerant to damage. This combination of mechanical properties has led to research into cuttlebone-inspired biomimetic ceramic foams.[18] In addition, due to its mechanical properties, cuttlebone has been used as scaffolding in superconductors[19] and tissue engineering applications.[20] The light weight of the cuttlebone derives from its high porosity (over 90% by volume).[17] The stiffness of the cuttlebone arises from the chambered structure composition of approximately 95% aragonite (a stiff material) and 5% organic material.[17] Since the stiffness of a composite will be dominated by the material with the largest volume fraction, the cuttlebone is also stiff. The specific stiffness of the cuttlebone in one species was measured to be as high as 8.4 [(MN)m/kg].[17] The most intriguing property of cuttlebone is its ability to tolerate damage given that aragonite is a brittle material. The high tolerance to damage can be linked to the cuttlebone's unique microstructure.[18]
Deformation process
[edit]Due to the marine lifestyle of the cuttlefish, the cuttlebone must be capable of both withstanding large compressive forces from the water while avoiding sudden brittle failure. The cuttlebone of some species under compression has demonstrated a specific energy on par with some advanced foams made from more compliant materials such as metals and polymers.[17] The high energy absorption is a result of several factors.
The failure of the cuttlebone occurs in three distinct stages: local crack formation, crack expansion, and densification.[17] Crack formation typically occurs in the middle of the vertical walls in the chambered structure of the cuttlebone.[17] The location of crack formation is controlled by the waviness in the corrugated structure of the walls. The waviness of the walls in the cuttlebone provides an optimized balance between stiffness and brittleness of the overall structure.[18] This wavy structure inhibits crack propagation, increasing the energy input necessary for failure. After sufficient damage has occurred to the walls of the cuttlebone, a process known as densification occurs whereby the walls gradually compact while fracture continues.[17] Significant energy is dissipated in the continued cracking of the walls while densification is occurring. It has also been observed that under compressive stresses, the horizontally layered chambers of the cuttlebone will fail sequentially. While one chamber is undergoing fracture and densification, the other chambers will not deform until the septum between the chambers has been penetrated.[17] The septum is significantly stronger than the vertical walls due to its "plywood" structure further increasing the total energy needed for complete structural failure of the cuttlebone.
See also
[edit]Explanatory footnotes
[edit]- ^ Jewelers prepare cuttlebone for use as a mold by cutting it in half and rubbing the two sides together until they fit flush against one another. Then the casting can be done by carving a design into the cuttlebone, adding the necessary sprue, melting the metal in a separate pouring crucible, and pouring the molten metal into the mold through the sprue. Finally, the sprue is sawed off and the finished piece is polished.
References
[edit]- ^ Fuchs, D.; Engeser, T.; Keupp, H. (2007). "Gladius shape variation in coleoid cephalopod Trachyteuthis from the upper Jurassic nusplingen and Solnhofen plattenkalks" (PDF). Acta Palaeontologica Polonica. 52 (3): 575–589.
- ^ Rexfort, A.; Mutterlose, J. (2006). "Stable isotope records from Sepia officinalis — a key to understanding the ecology of belemnites?". Earth and Planetary Science Letters. 247 (3–4): 212. Bibcode:2006E&PSL.247..212R. doi:10.1016/j.epsl.2006.04.025.
- ^ Norman, M.D. (2000). Cephalopods: A world guide. Conch Books.
- ^ a b "Uses for cuttlebone. The time when it was used as a medicine (1912)". Alton Evening Telegraph. 3 October 1912. p. 7. Retrieved 21 January 2016.
- ^ "Do you know this?". The World's News. 8 July 1950. p. 26. Retrieved 21 January 2016.
- ^ "Wesleyan anniversary". Portland Guardian and Normanby General Advertiser. 17 October 1872. p. 2. Retrieved 21 January 2016.
- ^ "Carnival at Norwood". Evening Journal. 24 October 1898. p. 3. Retrieved 21 January 2016.
- ^ "Eleanor Barbour's pages for country women". Chronicle. 16 July 1942. p. 26. Retrieved 21 January 2016.
- ^ "Note book cuttlefish". The Register News-Pictorial. 17 May 1930. p. 3S. Retrieved 21 January 2016.
- ^ "Models from cuttle-fish". The Age. Interesting Hobbies. 30 June 1950. p. 5S. Retrieved 21 January 2016.
- ^ "Back to semaphore celebrations". Port Adelaide News. 13 December 1929. p. 3. Retrieved 21 January 2016.
- ^ "Out among the people". The Advertiser. 12 May 1943. p. 6. Retrieved 21 January 2016.
- ^ Norman, M.D.; Reid, A. (2000). A Guide to Squid, Cuttlefish, and Octopuses of Australasia. CSIRO Publishing.
- ^ Zhu, X. D.; Luo, J. Y.; Kong, D. D.; Wu, J. J.; Sheng, P.; Yang, M. H. (2019). "海螵蛸中砷形态分析及限量标准研究 - 中国知网" [Analysis of arsenic speciation in Endoconcha Sepiae and research on its limit standard]. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica. 44 (23): 5065–5071. doi:10.19540/j.cnki.cjcmm.20190903.201. PMID 32237338.
generally contains high concentration of arsenic
- ^ Ferraz, E.; Gamelas, J.A.F.; Coroado, J.; Monteiro, C.; Rocha, F. (20 July 2020). "Exploring the potential of cuttlebone waste to produce building lime". Materiales de Construcción. 70 (339): 225. doi:10.3989/mc.2020.15819. hdl:10400.26/38428. ISSN 1988-3226.
- ^ a b c Checa, Antonio G.; Cartwright, Julyan H. E.; Sánchez-Almazo, Isabel; Andrade, José P.; Ruiz-Raya, Francisco (September 2015). "The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor". Scientific Reports. 5 (1): 11513. arXiv:1506.08290. Bibcode:2015NatSR...511513C. doi:10.1038/srep11513. ISSN 2045-2322. PMC 4471886. PMID 26086668.
- ^ a b c d e f g h i j k Yang, Ting; Jia, Zian; Chen, Hongshun; Deng, Zhifei; Liu, Wenkun; Chen, Liuni; Li, Ling (22 September 2020). "Mechanical design of the highly porous cuttlebone: A bioceramic hard buoyancy tank for cuttlefish". Proceedings of the National Academy of Sciences. 117 (38): 23450–23459. Bibcode:2020PNAS..11723450Y. doi:10.1073/pnas.2009531117. ISSN 0027-8424. PMC 7519314. PMID 32913055.
- ^ a b c "Cuttlebone's microstructure sits at a 'sweet spot'". ScienceDaily. Retrieved 14 May 2021.
- ^ Culverwell, Emily; Wimbush, Stuart C.; Hall, Simon R. (2008). "Biotemplated synthesis of an ordered macroporous superconductor with high critical current density using a cuttlebone template". Chem. Commun. (9): 1055–1057. doi:10.1039/B715368F. ISSN 1359-7345. PMID 18292888.
- ^ Kannan, S.; Rocha, J.H.G.; Agathopoulos, S.; Ferreira, J.M.F. (March 2007). "Fluorine-substituted hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones". Acta Biomaterialia. 3 (2): 243–249. doi:10.1016/j.actbio.2006.09.006. PMID 17127113.
- Neige, P. (2003). "Combining disparity with diversity to study the biogeographic pattern of Sepiidae" (PDF). Berliner Paläobiologische Abhandlungen. 3: 189–197.
External links
[edit] Media related to Cuttlebone at Wikimedia Commons
Media related to Cuttlebone at Wikimedia Commons