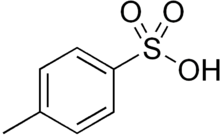
p-Toluenesulfonic acid
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Methylbenzene-1-sulfonic acid | |
| Other names
4-Methylbenzenesulfonic acid
Tosylic acid tosic acid PTSA | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.891 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H8O3S | |
| Molar mass | 172.20 g/mol (anhydrous) 190.22 g/mol (monohydrate) |
| Appearance | colorless (white) solid |
| Density | 1.24 g/cm3 |
| Melting point | 38 °C (100 °F; 311 K) (anhydrous)[2] 103 to 106 °C (217 to 223 °F; 376 to 379 K) (monohydrate) |
| Boiling point | 140 °C (284 °F; 413 K) at 20 mmHg |
| 67 g/100 mL | |
| Acidity (pKa) | −2.8 (water),[3] 8.5 (acetonitrile)[4] |
| Structure | |
| tetrahedral at S | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
skin irritant |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related sulfonic acids
|
Benzenesulfonic acid Sulfanilic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
p-Toluenesulfonic acid (PTSA or pTsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3C6H4SO3H. It is a white solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C6H4SO2– group is known as the tosyl group and is often abbreviated as Ts or Tos. Most often, TsOH refers to the monohydrate, TsOH.H2O.
As with other sulfonic acids, TsOH is a strong organic acid. It is about one million times stronger than benzoic acid. It is one of the few strong acids that is solid and, hence, conveniently weighed. Also, unlike some strong mineral acids (especially nitric acid, sulfuric acid, and perchloric acid), TsOH is non-oxidizing.[citation needed]
Preparation and handling
TsOH is prepared on an industrial scale by the sulfonation of toluene. It hydrates readily. Common impurities include benzenesulfonic acid and sulfuric acid. Impurities can be removed by recrystallization from its concentrated aqueous solution followed by azeotropic drying with toluene.[5]
TsOH finds use in organic synthesis as an "organic-soluble" acid catalyst. Examples of uses include:
- Acetalization of an aldehyde.[6]
- Esterification of carboxylic acids.[7]
- Transesterification of an ester.[8]
Tosylate esters

Tosylate esters are used as alkylating agents because the tosyl group is electron-withdrawing, which makes the tosylate anion a good leaving group. The tosyl group is also a protecting group for alcohols and amines, prepared by combining the alcohol with 4-toluenesulfonyl chloride, usually in an aprotic solvent, often pyridine, the basicity of which activates the reaction.[9] Toluenesulfonate esters undergo nucleophilic attack or elimination. Reduction of tosylate esters gives the hydrocarbon. Thus, tosylation followed by reduction allows for the deoxygenation of alcohols.
Reactions
- TsOH may be converted to p-toluenesulfonic anhydride by heating with phosphorus pentoxide.[10]
- When TsOH is heated with acid and water, a hydrolysis reaction takes place and toluene is formed:
- CH3C6H4SO3H + H2O → C6H5CH3 + H2SO4
This reaction is general for aryl sulfonic acids, but the rate at which it occurs depends upon the structure of the acid, the temperature and the nature of the catalyzing acid. For example, TsOH is unaffected by cold concentrated hydrochloric acid, but hydrolyzes when heated to 186 °C in concentrated phosphoric acid.[11][12]
See also
References
- ^ Merck Index, 11th Edition, 9459.
- ^ Armarego, Wilfred (2003). Purification of Laboratory Chemicals. Elsevier Science. p. 370. ISBN 0-7506-7571-3.
- ^ Guthrie, J. P. Hydrolysis of esters of oxy acids: pKa values for strong acids. Can. J. Chem. 1978, 56, 2342-2354.
- ^ Eckert, F.; Leito, I.; Kaljurand, I.; Kütt, A.; Klamt, A.; Diedenhofen, M. Prediction of Acidity in Acetonitrile Solution with COSMO-RS. J. Comput. Chem. 2009, 30, 799-810. doi:10.1002/jcc.21103
- ^ Perrin, D. D.; Armarego, W. L. F. (1988). Purification of Laboratory Chemicals. Oxford: Pergamon Press.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ H. Griesser, H.; Öhrlein, R.; Schwab, W.; Ehrler, R.; Jäger, V. (2004). "3-Nitropropanal, 3-Nitropropanol, and 3-Nitropropanal Dimethyl Acetal". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 10, p. 577. - ^ Furuta, K. Gao, Q.-z.; Yamamoto, H. (1998). "Chiral (Acyloxy)borane Complex-catalyzed Asymmetric Diels-Alder Reaction: (1R)-1,3,4-Trimethyl-3-cyclohexene-1-carboxaldehyde". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 9, p. 722. - ^ Imwinkelried, R.; Schiess, M.; Seebach, D. (1993). "Diisopropyl (2S,3S)-2,3-O-isopropylidenetartrate". Organic Syntheses
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 8, p. 201. - ^ "Nucleophilic Substitution".
- ^ L. Field and J. W. McFarland (1963). "p-Toluenesulfonic Anhydride". Organic Syntheses; Collected Volumes, vol. 4, p. 940.
- ^ C. M. Suter (1944). The Organic Chemistry of Sulfur. New York: John Wiley & Sons. pp. 387–388.
- ^ J. M. Crafts (1901). "Catalysis in concentrated solutions". J. Am. Chem. Soc. 23: 236–249.
