Phloretin
| |
| Names | |
|---|---|
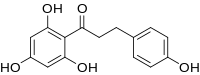
| Preferred IUPAC name
3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one | |
| Other names
Dihydronaringenin
Phloretol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.000.444 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H14O5 | |
| Molar mass | 274.272 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Phloretin is a dihydrochalcone, a type of natural phenol. It can be found in apple tree leaves[1] and the Manchurian apricot.[2]
Metabolism
[edit]In rats, ingested phlorizin is converted into phloretin by hydrolytic enzymes in the small intestine.[3][4] Phloretin hydrolase hydrolyses phloretin into phloretic acid and phloroglucinol.
Pharmacological research
[edit]In an animal model, phloretin inhibited active transport of glucose into cells by SGLT1 and SGLT2, though the inhibition is weaker than by its glycoside phlorizin.[5] An important effect of this is the inhibition of glucose absorption by the small intestine[4] and the inhibition of renal glucose reabsorption.[3] Phloretin also inhibits a variety of urea transporters.[6][7] It induces urea loss and diuresis when coupled with high protein diets. Phloretin has been found to inhibit weight gain and improve metabolic homeostasis in mice fed with high-fat diet.[8] Phloretin inhibits aquaporin 9 (AQP9) on mouse hepatocytes.[9]
Nanoparticle Synthesis
[edit]Phloretin functionalized gold-nanoparticles (Pht-GNPs) were synthesized using a single-step synthesis method and tested for its anticancer activity. Pht-GNPs showed significant cancer cell toxicities compared to free phloretin.[10]
Glycosides
[edit]- Phlorizin is the 2'-glucoside of phloretin
- Naringin dihydrochalcone is a diglycoside of phloretin
See also
[edit]References
[edit]- ^ Picinelli A.; Dapena E.; Mangas J. J. (1995). "Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study". Journal of Agricultural and Food Chemistry. 43 (8): 2273–2278. doi:10.1021/jf00056a057.
- ^ "Manchurian Apricot (Prunus armeniaca var. mandshurica)" (PDF). North Dakota State University. Retrieved January 30, 2014.
- ^ a b Idris, I.; Donnelly, R. (2009). "Sodium-glucose co-transporter-2 inhibitors: An emerging new class of oral antidiabetic drug". Diabetes, Obesity and Metabolism. 11 (2): 79–88. doi:10.1111/j.1463-1326.2008.00982.x. PMID 19125776.
- ^ a b Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. (2001). "Bioavailability of phloretin and phloridzin in rats". The Journal of Nutrition. 131 (12): 3227–3230. doi:10.1093/jn/131.12.3227. PMID 11739871.
- ^ Chan, Stephen S.; William D. Lotspeich (1962-12-01). "Comparative effects of phlorizin and phloretin on glucose transport in the cat kidney". American Journal of Physiology. Legacy Content. 203 (6): 975–979. doi:10.1152/ajplegacy.1962.203.6.975. ISSN 0002-9513. PMID 14019989. Retrieved 2012-10-21.
- ^ Fenton, Robert A.; Chung-Lin Chou; Gavin S. Stewart; Craig P. Smith; Mark A. Knepper (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode:2004PNAS..101.7469F. doi:10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Shayakul, Chairat; Hiroyasu Tsukaguchi; Urs V. Berger; Matthias A. Hediger (2001-03-01). "Molecular characterization of a novel urea transporter from kidney inner medullary collecting ducts". American Journal of Physiology. Renal Physiology. 280 (3): F487–F494. doi:10.1152/ajprenal.2001.280.3.f487. ISSN 1931-857X. PMID 11181411. S2CID 22143248. Archived from the original on 2016-03-04. Retrieved 2012-10-21.
- ^ Alsanea, Sary; Gao, Mingming; Liu, Dexi (May 2017). "Phloretin Prevents High-Fat Diet-Induced Obesity and Improves Metabolic Homeostasis". The AAPS Journal. 19 (3): 797–805. doi:10.1208/s12248-017-0053-0. ISSN 1550-7416. PMID 28197827. S2CID 3638970.
- ^ Fenton, Robert A.; Chou, Chung-Lin; Stewart, Gavin S.; Smith, Craig P.; Knepper, Mark A. (2004-05-11). "Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct". Proceedings of the National Academy of Sciences of the United States of America. 101 (19): 7469–7474. Bibcode:2004PNAS..101.7469F. doi:10.1073/pnas.0401704101. ISSN 0027-8424. PMC 409942. PMID 15123796.
- ^ Payne NJ, Badwaik VD, Waghwani HK, Moolani HV, Tockstein S, Thompson DH, Dakshinamurthy R (March 2018). "Development of dihydrochalcone-functionalized gold nanoparticles for augmented antineoplastic activity". International Journal of Nanomedicine. 13: 1917–1926. doi:10.2147/IJN.S143506. PMC 5880570. PMID 29636609.
