Oxalic acid: Difference between revisions
→Niche uses: Whitening clay for ceramics Tag: Disambiguation links added |
→Production by fungi: Industrial production considered |
||
| Line 172: | Line 172: | ||
===Production by fungi=== |
===Production by fungi=== |
||
Many soil fungus species secrete oxalic acid, resulting in greater solubility of metal cations, increased availability of certain soil nutrients, and can lead to the formation of calcium oxalate crystals.<ref>{{cite journal |last1=Dutton |first1=Martin V. |last2=Evans |first2=Christine S. |title=Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment |journal=Canadian Journal of Microbiology |date=1 September 1996 |volume=42 |issue=9 |pages=881–895 |doi=10.1139/m96-114}}</ref><ref>{{cite journal|author1-link=Geoffrey Michael Gadd |last1=Gadd |first1=Geoffrey M. |title=Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes |journal=Advances in Microbial Physiology |date=1 January 1999 |volume=41 |pages=47–92 |publisher=Academic Press |language=en|doi=10.1016/S0065-2911(08)60165-4 |pmid=10500844 |isbn=9780120277414 }}</ref> |
Many soil fungus species secrete oxalic acid, resulting in greater solubility of metal cations, increased availability of certain soil nutrients, and can lead to the formation of calcium oxalate crystals.<ref>{{cite journal |last1=Dutton |first1=Martin V. |last2=Evans |first2=Christine S. |title=Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment |journal=Canadian Journal of Microbiology |date=1 September 1996 |volume=42 |issue=9 |pages=881–895 |doi=10.1139/m96-114}}</ref><ref>{{cite journal|author1-link=Geoffrey Michael Gadd |last1=Gadd |first1=Geoffrey M. |title=Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes |journal=Advances in Microbial Physiology |date=1 January 1999 |volume=41 |pages=47–92 |publisher=Academic Press |language=en|doi=10.1016/S0065-2911(08)60165-4 |pmid=10500844 |isbn=9780120277414 }}</ref> Some fungi such as ''[[Aspergillus niger]]'' have been extensively studied for the industrial production of oxalic acid<ref name=stra1994>Hermann Strasser, Wolfgang Burgstaller, Franz Schinner(1994): "High-yield production of oxalic acid for metal leaching processes by ''Aspergillus niger''". ''FEMS Microbiology Letters'', volume 119, issue 3, pages 365–370. {{doi|10.1111/j.1574-6968.1994.tb06914.x}}</ref>; however, those processes are not yet economically competitive with production from oil and gas.<ref name=tkacz2012>Jan S. Tkacz, Lene Lange (2012): ''Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine''. 445 pages. {{isbn|9781441988591}}</ref> |
||
===Other=== |
===Other=== |
||
Revision as of 22:32, 19 November 2021
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalic acid[1] | |||
| Systematic IUPAC name
Ethanedioic acid[1] | |||
| Other names
Wood bleach, Crab Acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 385686 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.123 | ||
| EC Number |
| ||
| 2208 | |||
| KEGG | |||
| MeSH | Oxalic+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 3261 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H2O4 | |||
| Molar mass | 90.034 g·mol−1 (anhydrous) 126.065 g·mol−1 (dihydrate) | ||
| Appearance | White crystals | ||
| Odor | Odorless | ||
| Density | 1.90 g·cm−3 (anhydrous, at 17 °C)[2] 1.653 g·cm−3 (dihydrate) | ||
| Melting point | 189 to 191 °C (372 to 376 °F; 462 to 464 K) 101.5 °C (214.7 °F; 374.6 K) dihydrate | ||
| 90–100 g/L (20 °C)[2] | |||
| Solubility | 237 g/L (15 °C) in ethanol 14 g/L (15 °C) in diethyl ether[3] | ||
| Vapor pressure | <0.001 mmHg (20 °C)[4] | ||
| Acidity (pKa) | 1.25, 4.14[5] | ||
| Conjugate base | Hydrogenoxalate | ||
| −60.05·10−6 cm3/mol | |||
| Pharmacology | |||
| QP53AG03 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Corrosive | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 166 °C (331 °F; 439 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published)
|
1000 mg/kg (dog, oral) 1400 mg/kg (rat) 7500 mg/kg (rat, oral)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1 mg/m3[4] | ||
REL (Recommended)
|
TWA 1 mg/m3 ST 2 mg/m3[4] | ||
IDLH (Immediate danger)
|
500 mg/m3[4] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
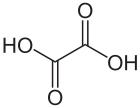
Oxalic acid is an organic acid with the IUPAC name ethanedioic acid and formula HO2C−CO2H. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods, but excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent[7] and its conjugate base, known as oxalate (C2O2−4), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula C2H2O4·2H2O.
History
The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel.[8] By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from its salt in sorrel.[9]
In 1776, Swedish chemists Carl Wilhelm Scheele and Torbern Olof Bergman[10] produced oxalic acid by reacting sugar with concentrated nitric acid; Scheele called the acid that resulted socker-syra or såcker-syra (sugar acid). By 1784, Scheele had shown that "sugar acid" and oxalic acid from natural sources were identical.[11]
In 1824, the German chemist Friedrich Wöhler obtained oxalic acid by reacting cyanogen with ammonia in aqueous solution.[12] This experiment may represent the first synthesis of a natural product.[13]
Preparation
Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. A variety of precursors can be used including glycolic acid and ethylene glycol.[14] A newer method entails oxidative carbonylation of alcohols to give the diesters of oxalic acid:
These diesters are subsequently hydrolyzed to oxalic acid. Approximately 120,000 tonnes are produced annually.[13]
Historically oxalic acid was obtained exclusively by using caustics, such as sodium or potassium hydroxide, on sawdust.[15] Pyrolysis of sodium formate (ultimately prepared from carbon monoxide), leads to the formation of sodium oxalate, easily converted to oxalic acid.
Laboratory methods
Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst.[16]
The hydrated solid can be dehydrated with heat or by azeotropic distillation.[17]
Developed in the Netherlands, an electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic acid;[18] this conversion uses carbon dioxide as a feedstock to generate oxalic acid.
Structure
Anhydrous oxalic acid exists as two polymorphs; in one the hydrogen-bonding results in a chain-like structure whereas the hydrogen bonding pattern in the other form defines a sheet-like structure.[19] Because the anhydrous material is both acidic and hydrophilic (water seeking), it is used in esterifications.
Reactions
Oxalic acid is relatively strong for a carboxylic acid:
| C2O4H2 ⇌ C2O4H− + H+ | pKa = 1.27 | |
| C2O4H− ⇌ C 2O2− 4 + H+ |
pKa = 4.27 |
Oxalic acid undergoes many of the reactions characteristic of other carboxylic acids. It forms esters such as dimethyl oxalate (m.p. 52.5 to 53.5 °C, 126.5 to 128.3 °F).[20] It forms an acid chloride called oxalyl chloride.
Oxalate, the conjugate base of oxalic acid, is an excellent ligand for metal ions, e.g. the drug oxaliplatin.
Oxalic acid and oxalates can be oxidized by permanganate in an autocatalytic reaction.[21]
Oxalic acid's pKa values vary in the literature from 1.25–1.46 and 3.81–4.40.[22][23][24] The 100th ed of the CRC, released in 2019 has values of 1.25 and 3.81.[25]
Occurrence
Biosynthesis
At least two pathways exist for the enzyme-mediated formation of oxalate. In one pathway, oxaloacetate, a component of the Krebs citric acid cycle, is hydrolyzed to oxalate and acetic acid by the enzyme oxaloacetase:[26]
2O2−
4 + CH
3CO−
2 + H+
It also arises from the dehydrogenation of glycolic acid, which is produced by the metabolism of ethylene glycol.
Occurrence in foods and plants
Early investigators isolated oxalic acid from wood-sorrel (Oxalis). Members of the spinach family and the brassicas (cabbage, broccoli, brussels sprouts) are high in oxalates, as are sorrel and umbellifers like parsley.[27] Rhubarb leaves contain about 0.5% oxalic acid, and jack-in-the-pulpit (Arisaema triphyllum) contains calcium oxalate crystals. Similarly, the Virginia creeper, a common decorative vine, produces oxalic acid in its berries as well as oxalate crystals in the sap, in the form of raphides. Bacteria produce oxalates from oxidation of carbohydrates.[13]
Plants of the genus Fenestraria produce optical fibers made from crystalline oxalic acid to transmit light to subterranean photosynthetic sites.[28]
Carambola, also known as starfruit, also contains oxalic acid along with caramboxin. Citrus juice contains small amounts of oxalic acid. Citrus fruits produced in organic agriculture contain less oxalic acid than those produced in conventional agriculture.[29]
The formation of naturally occurring calcium oxalate patinas on certain limestone and marble statues and monuments has been proposed to be caused by the chemical reaction of the carbonate stone with oxalic acid secreted by lichen or other microorganisms.[30][31]
Production by fungi
Many soil fungus species secrete oxalic acid, resulting in greater solubility of metal cations, increased availability of certain soil nutrients, and can lead to the formation of calcium oxalate crystals.[32][33] Some fungi such as Aspergillus niger have been extensively studied for the industrial production of oxalic acid[34]; however, those processes are not yet economically competitive with production from oil and gas.[35]
Other
Oxidized bitumen or bitumen exposed to gamma rays also contains oxalic acid among its degradation products. Oxalic acid may increase the leaching of radionuclides conditioned in bitumen for radioactive waste disposal.[36]
Biochemistry
The conjugate base of oxalic acid is the hydrogenoxalate anion, and its conjugate base (oxalate) is a competitive inhibitor of the lactate dehydrogenase (LDH) enzyme.[37] LDH catalyses the conversion of pyruvate to lactic acid (end product of the fermentation (anaerobic) process) oxidising the coenzyme NADH to NAD+ and H+ concurrently. Restoring NAD+ levels is essential to the continuation of anaerobic energy metabolism through glycolysis. As cancer cells preferentially use anaerobic metabolism (see Warburg effect) inhibition of LDH has been shown to inhibit tumor formation and growth,[38] thus is an interesting potential course of cancer treatment.
Applications
About 25% of produced oxalic acid will be used as a mordant in dyeing processes. It is also used in bleaches, especially for pulpwood, and for rust removal and other cleaning, in baking powder,[13] and as a third reagent in silica analysis instruments.
Cleaning
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent). Its utility in rust removal agents is due to its forming a stable, water-soluble salt with ferric iron, ferrioxalate ion. The cleaning product Zud contains oxalic acid.[39]
Oxalic acid is also widely used as a wood bleach, most often in its crystalline form to be mixed with water to its proper dilution for use.
Extractive metallurgy
Oxalic acid is an important reagent in lanthanide chemistry. Hydrated lanthanide oxalates form readily in very strongly acidic solutions in a densely crystalline, easily filtered form, largely free of contamination by nonlanthanide elements. Thermal decomposition of these oxalates gives the oxides, which is the most commonly marketed form of these elements.
Niche uses

Oxalic acid is used by some beekeepers as a miticide against the parasitic varroa mite.[40]
Dilute solutions (0.05–0.15 M) of oxalc acid can be used to remove iron from clays such as kaolinite to produce light-colored ceramics.[41]
Oxalic acid is used to clean minerals.[42][43]
Oxalic acid is sometimes used in the aluminum anodizing process, with or without sulfuric acid.[citation needed] Compared to sulfuric acid anodizing, the coatings obtained are thinner and exhibit lower surface roughness.
Oxalic acid is an ingredient in some tooth whitening products.
Content in food items
| Vegetable | Oxalic acid (g/100 g)a[clarification needed] |
|---|---|
| Amaranth | 1.09 |
| Asparagus | 0.13 |
| Beans, snap | 0.36 |
| Beet leaves | 0.61 |
| Beetroot | 0.06[45] |
| Broccoli | 0.19 |
| Brussels sprouts | 0.02[45] |
| Cabbage | 0.10 |
| Carrot | 0.50 |
| Cassava | 1.26 |
| Cauliflower | 0.15 |
| Celery | 0.19 |
| Chicory | 0.2 |
| Chives | 1.48 |
| Collards | 0.45 |
| Coriander | 0.01 |
| Corn, sweet | 0.01 |
| Cucumber | 0.02 |
| Eggplant | 0.19 |
| Endive | 0.11 |
| Garlic | 0.36 |
| Kale | 0.02 |
| Lettuce | 0.33 |
| Okra | 0.05 |
| Onion | 0.05 |
| Parsley | 1.70 |
| Parsnip | 0.04 |
| Pea | 0.05 |
| Bell pepper | 0.04 |
| Potato | 0.05 |
| Purslane | 1.31 |
| Radish | 0.48 |
| Rhubarb leaves | 0.52[46] |
| Rutabaga | 0.03 |
| Spinach | 0.97 (ranges from .65 to 1.3 grams per 100 grams on fresh weight basis)[47] |
| Squash | 0.02 |
| Sweet potato | 0.24 |
| Swiss Chard, green | 0.96 [45] |
| Tomato | 0.05 |
| Turnip | 0.21 |
| Turnip greens | 0.05 |
| Watercress | 0.31 |
Toxicity
Oxalic acid in concentrated form can have harmful effects through contact and if ingested. It is not identified as mutagenic or carcinogenic, although there is a study suggesting it might cause breast cancer;[48] there is a possible risk of congenital malformation in the fetus; may be harmful if inhaled, and is extremely destructive to tissue of mucous membranes and upper respiratory tract; harmful if swallowed; harmful to and destructive of tissue and causes burns if absorbed through the skin or is in contact with the eyes. Symptoms and effects include a burning sensation, cough, wheezing, laryngitis, shortness of breath, spasm, inflammation and edema of the larynx, inflammation and edema of the bronchi, pneumonitis, pulmonary edema.[49]
In humans, ingested oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg.[50] It has been reported that the lethal oral dose is 15 to 30 grams.[51] The toxicity of oxalic acid is due to kidney failure caused by precipitation of solid calcium oxalate.[52]
Oxalate may enter cells where it is known to cause mitochondrial dysfunction.[53]
Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.
Kidney stones
The vast majority kidney stones, 76%, are composed of the calcium salt of oxalic acid.[54] Oxalic acid can also cause joint pain by formation of similar precipitates in the joints. Calcium hydroxide (slaked lime) decreases urinary oxalate in both humans and rats.[55] Ingesting both calcium containing foods, such as milk, with food high in oxalic acid, cause the formation of calcium oxalate in the stomach, which is not absorbed into the body.
Between 1% and 15% of people globally are affected by kidney stones at some point in their lives.[56][57] In 2015, they caused about 16,000 deaths worldwide.[58]
Notes
^a Unless otherwise cited, all measurements are based on raw vegetable weights with original moisture content.
References
- ^ a b "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Radiant Agro Chem. "Oxalic Acid MSDS". Archived from the original on 2011-07-15. Retrieved 2012-02-02.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0474". National Institute for Occupational Safety and Health (NIOSH).
- ^ Bjerrum, Jannik; Sillén, Lars Gunnar; Schwarzenbach, Gerold Karl; Anderegg, Giorgio (1958). Stability constants of metal-ion complexes, with solubility products of inorganic substances. London: Chemical Society.
- ^ "Oxalic acid". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 2005. pp. 17624/28029. doi:10.1002/14356007. ISBN 9783527306732.
- ^ See:
- Herman Boerhaave, Elementa Chemiae (Basil, Switzerland: Johann Rudolph Im-hoff, 1745), volume 2, pp. 35-38. (in Latin) From p. 35: "Processus VII. Sal nativum plantarum paratus de succo illarum recens presso. Hic Acetosae." (Procedure 7. A natural salt of plants prepared from their freshly pressed juice. This [salt obtained] from sorrel.)
- Henry Enfield Roscoe and Carl Schorlemmer, ed.s, A Treatise on Chemistry (New York, New York: D. Appleton and Co., 1890), volume 3, part 2, p. 105.
- See also Wikipedia's articles "Oxalis acetosella" and "Potassium hydrogen oxalate".
- ^ See:
- François Pierre Savary, Dissertatio Inauguralis De Sale Essentiali Acetosellæ [Inaugural dissertation on the essential salt of wood sorrel] (Jean François Le Roux, 1773). (in Latin) Savary noticed that when he distilled sorrel salt (potassium hydrogen oxalate), crystals would sublimate onto the receiver. From p. 17: "Unum adhuc circa liquorem acidum, quem sal acetosellae tam sincerissimum a nobis paratum quam venale destillatione fundit phoenomenon erit notandum, nimirum quod aliquid ejus sub forma sicca crystallina lateribus excipuli accrescat, ..." (One more [thing] will be noted regarding the acid liquid, which furnished for us sorrel salt as pure as commercial distillations, [it] produces a phenomenon, that evidently something in dry, crystalline form grows on the sides of the receiver, ...) These were crystals of oxalic acid.
- Leopold Gmelin with Henry Watts, trans., Hand-book of Chemistry (London, England: Cavendish Society, 1855), volume 9, p. 111.
- ^ See:
- Torbern Bergman with Johan Afzelius (1776) Dissertatio chemica de acido sacchari [Chemical dissertation on sugar acid] (Uppsala, Sweden: Edman, 1776).
- Torbern Bergman, Opuscula Physica et Chemica, (Leipzig (Lipsia), (Germany): I.G. Müller, 1776), volume 1, "VIII. De acido Sacchari," pp. 238-263.
- ^ Carl Wilhelm Scheele (1784) "Om Rhabarber-jordens bestånds-delar, samt sått at tilreda Acetosell-syran" (On rhubarb-earth's constituents, as well as ways of preparing sorrel-acid), Kungliga Vetenskapsakademiens Nya Handlingar [New Proceedings of the Royal Academy of Science], 2nd series, 5 : 183-187. (in Swedish) From p. 187: "Således finnes just samma syra som vi genom konst af socker med tilhjelp af salpeter-syra tilreda, redan förut af naturen tilredd uti o̊rten Acetosella." (Thus it is concluded [that] the very same acid as we prepare artificially by means of sugar with the help of nitric acid, [was] previously prepared naturally in the herb acetosella [i.e., sorrel].)
- ^ See:
- F. Wöhler (1824) "Om några föreningar af Cyan" (On some compounds of cyanide), Kungliga Vetenskapsakademiens Handlingar [Proceedings of the Royal Academy of Science], pp. 328-333. (in Swedish)
- Reprinted in German as: F. Wöhler (1825) "Ueber Cyan-Verbindungen" (On cyanide compounds), Annalen der Physik und Chemie, 2nd series, 3 : 177-182.
- ^ a b c d Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a18_247.
- ^ Eiichi, Yonemitsu; Tomiya, Isshiki; Tsuyoshi, Suzuki and Yukio, Yashima "Process for the production of oxalic acid", U.S. patent 3,678,107, priority date March 15, 1969
- ^ Von Wagner, Rudolf (1897). Manual of chemical technology. New York: D. Appleton & Co. p. 499.
- ^ Practical Organic Chemistry by Julius B. Cohen, 1930 ed. preparation #42
- ^ Clarke H. T.;. Davis, A. W. (1941). "Oxalic acid (anhydrous)". Organic Syntheses: 421
{{cite journal}}: CS1 maint: multiple names: authors list (link); Collected Volumes, vol. 1. - ^ Bouwman, Elisabeth; Angamuthu, Raja; Byers, Philip; Lutz, Martin; Spek, Anthony L. (July 15, 2010). "Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex". Science. 327 (5393): 313–315. Bibcode:2010Sci...327..313A. CiteSeerX 10.1.1.1009.2076. doi:10.1126/science.1177981. PMID 20075248. S2CID 24938351.
- ^ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ^ Bowden, E. (1943). "Methyl oxalate". Organic Syntheses: 414; Collected Volumes, vol. 2.
- ^ Kovacs K. A.; Grof P.; Burai L.; Riedel M. (2004). "Revising the mechanism of the permanganate/oxalate reaction". Journal of Physical Chemistry A. 108 (50): 11026–11031. Bibcode:2004JPCA..10811026K. doi:10.1021/jp047061u.
- ^ Bjerrum, J., et al. (1958) Stability Constants, Chemical Society, London.
- ^ Haynes, W. M. (Ed.). (2014). CRC Handbook of Chemistry and Physics, 95th Edition (95 edition). Boca Raton; London; New York: CRC Press.
- ^ Clayton, G. D. and Clayton, F. E. (eds.). Patty's Industrial Hygiene and Toxicology, Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981–1982., p. 4936
- ^ Rumble, J. (Ed.). (2019). CRC Handbook of Chemistry and Physics, 100th Edition (100 edition). CRC Press.
- ^ Dutton, M. V.; Evans, C. S. (1996). "Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment". Canadian Journal of Microbiology. 42 (9): 881–895. doi:10.1139/m96-114..
- ^ Rombauer, Rombauer Becker, and Becker (1931/1997). Joy of Cooking, p.415. ISBN 0-684-81870-1.
- ^ Attenborough, David. "Surviving." The Private Life of Plants: A Natural History of Plant Behaviour. Princeton, NJ: Princeton UP, 1995. 265+. "OpenLibrary.org: The Private Life of Plants" Print.
- ^ Duarte, A.; Caixeirinho, D.; Miguel, M.; Sustelo, V.; Nunes, C.; Fernandes, M.; Marreiros, A. (2012). "Organic Acids Concentration in Citrus Juice from Conventional versus Organic Farming". Acta Horticulturae. 933 (933): 601–606. doi:10.17660/ActaHortic.2012.933.78. hdl:10400.1/2790.
- ^ Sabbioni, Cristina; Zappia, Giuseppe (2016). "Oxalate patinas on ancient monuments: The biological hypothesis". Aerobiologia. 7: 31–37. doi:10.1007/BF02450015. S2CID 85017563.
- ^ Frank-Kamemetskaya, Olga; Rusakov, Alexey; Barinova, Ekaterina; Zelenskaya, Marina; Vlasov, Dmitrij (2012). "The Formation of Oxalate Patina on the Surface of Carbonate Rocks Under the Influence of Microorganisms". Proceedings of the 10th International Congress for Applied Mineralogy (ICAM). pp. 213–220. doi:10.1007/978-3-642-27682-8_27. ISBN 978-3-642-27681-1.
- ^ Dutton, Martin V.; Evans, Christine S. (1 September 1996). "Oxalate production by fungi: its role in pathogenicity and ecology in the soil environment". Canadian Journal of Microbiology. 42 (9): 881–895. doi:10.1139/m96-114.
- ^ Gadd, Geoffrey M. (1 January 1999). "Fungal Production of Citric and Oxalic Acid: Importance in Metal Speciation, Physiology and Biogeochemical Processes". Advances in Microbial Physiology. 41. Academic Press: 47–92. doi:10.1016/S0065-2911(08)60165-4. ISBN 9780120277414. PMID 10500844.
- ^ Hermann Strasser, Wolfgang Burgstaller, Franz Schinner(1994): "High-yield production of oxalic acid for metal leaching processes by Aspergillus niger". FEMS Microbiology Letters, volume 119, issue 3, pages 365–370. doi:10.1111/j.1574-6968.1994.tb06914.x
- ^ Jan S. Tkacz, Lene Lange (2012): Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine. 445 pages. ISBN 9781441988591
- ^ EPJ Web of Conferences
- ^ Novoa, William; Alfred Winer; Andrew Glaid; George Schwert (1958). "Lactic Dehydrogenase V. inhibition by Oxamate and Oxalate". Journal of Biological Chemistry. 234 (5): 1143–8. doi:10.1016/S0021-9258(18)98146-9. PMID 13654335.
- ^ Le, Anne; Charles Cooper; Arvin Gouw; Ramani Dinavahi; Anirban Maitra; Lorraine Deck; Robert Royer; David Vander Jagt; Gregg Semenza; Chi Dang (14 December 2009). "Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression". Proceedings of the National Academy of Sciences. 107 (5): 2037–2042. doi:10.1073/pnas.0914433107. PMC 2836706. PMID 20133848.
- ^ "Oxalic Acid Best Treatment For Getting Rid Of Concrete Stains". The Hartford Courant. 7 August 2011. Retrieved 14 January 2021.
- ^ Yu-Lun Lisa Fu (2008). Exploring New Methods for Varroa Mite Control. Michigan State University.
- ^ Sung Oh Lee, Tam Tran, Byoung Hi Jung, Seong Jun Kim, and Myong Jun Kim (2007): "Dissolution of iron oxide using oxalic acid". Hydrometallurgy, volume 87, issues 3–4. pages 91-99. doi:10.1016/j.hydromet.2007.02.005
- ^ Jackson, Faith. "Quartz Crystal Cleaning" Archived 2013-10-29 at the Wayback Machine. bluemooncrystals.com
- ^ "Rock Currier – Cleaning Quartz". mindat.org
- ^ All data not specifically annotated is from Agriculture Handbook No. 8-11, Vegetables and Vegetable Products, 1984. ("Nutrient Data : Oxalic Acid Content of Selected Vegetables". ars.usda.gov)
- ^ a b c Chai, Weiwen; Liebman, Michael (2005). "Effect of Different Cooking Methods on Vegetable Oxalate Content". Journal of Agricultural and Food Chemistry. 53 (8): 3027–30. doi:10.1021/jf048128d. PMID 15826055.
- ^ Pucher, GW; Wakeman, AJ; Vickery, HB (1938). "The organic acids of rhubarb (Rheum hybridium). III. The behavior of the organic acids during culture of excised leaves". Journal of Biological Chemistry. 126 (1): 43. doi:10.1016/S0021-9258(18)73892-1.
- ^ Durham, Sharon. "Making Spinach with Low Oxalate Levels". AgResearch Magazine. No. January 2017. United States Department of Agriculture. Retrieved 26 June 2017.
The scientists analyzed oxalate concentrations in 310 spinach varieties—300 USDA germplasm accessions and 10 commercial cultivars. "These spinach varieties and cultivars displayed oxalate concentrations from 647.2 to 1286.9 mg/100 g on a fresh weight basis," says Mou.
- ^ Castellaro, Andrés M.; Tonda, Alfredo; Cejas, Hugo H.; Ferreyra, Héctor; Caputto, Beatriz L.; Pucci, Oscar A.; Gil, German A. (2015-10-22). "Oxalate induces breast cancer". BMC Cancer. 15: 761. doi:10.1186/s12885-015-1747-2. ISSN 1471-2407. PMC 4618885. PMID 26493452.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Oxalic acid dihydrate. MSDS. sigmaaldrich.com
- ^ "Oxalic Acid Material Safety Data Sheet" (PDF). Radiant Indus Chem. Archived from the original (PDF) on 2014-05-20. Retrieved 2014-05-20.
- ^ "CDC – Immediately Dangerous to Life or Health Concentrations (IDLH): Oxalic acid – NIOSH Publications and Products". cdc.gov
- ^ EMEA Committee for veterinary medicinal products, oxalic acid summary report, December 2003
- ^ Patel, Mikita; Yarlagadda, Vidhush; Adedoyin, Oreoluwa; Saini, Vikram; Assimos, Dean G.; Holmes, Ross P.; Mitchell, Tanecia (May 2018). "Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line". Redox Biology. 15: 207–215. doi:10.1016/j.redox.2017.12.003. PMC 5975227. PMID 29272854.
- ^ Singh, Prince; Enders, Felicity T.; Vaughan, Lisa E.; Bergstralh, Eric J.; Knoedler, John J.; Krambeck, Amy E.; Lieske, John C.; Rule, Andrew D. (October 2015). "Stone Composition Among First-Time Symptomatic Kidney Stone Formers in the Community". Mayo Clinic Proceedings. 90 (10): 1356–1365. doi:10.1016/j.mayocp.2015.07.016. PMC 4593754. PMID 26349951.
- ^ https://www.researchgate.net/publication/287536542_Effect_of_addition_of_calcium_hydroxide_to_foods_rich_in_oxalic_acid_on_calcium_and_oxalic_acid_metabolism
- ^ Morgan, Monica S C; Pearle, Margaret S (2016). "Medical management of renal stones". BMJ. 352: i52. doi:10.1136/bmj.i52. ISSN 1756-1833. PMID 26977089. S2CID 28313474.
- ^ Abufaraj, Mohammad; Xu, Tianlin; Cao, Chao; Waldhoer, Thomas; Seitz, Christian; d'Andrea, David; Siyam, Abdelmuez; Tarawneh, Rand; Fajkovic, Harun; Schernhammer, Eva; Yang, Lin; Shariat, Shahrokh F. (2020-09-06). "Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007–2018 Data". European Urology Focus. doi:10.1016/j.euf.2020.08.011. ISSN 2405-4569. PMID 32900675. S2CID 221572651.
- ^ Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
External links
- Oxalic acid MS Spectrum
- International Chemical Safety Card 0529
- NIOSH Guide to Chemical Hazards (CDC)
- Table: Oxalic acid content of selected vegetables (USDA)
- Alternative link: Table: Oxalic Acid Content of Selected Vegetables (USDA)
- About rhubarb poisoning (The Rhubarb Compendium)
- Oxalosis & Hyperoxaluria Foundation (OHF) The Oxalate Content of Food 2008 (PDF)
- Oxalosis & Hyperoxaluria Foundation (OHF) Diet Information
- Calculator: Water and solute activities in aqueous oxalic acid