Polychlorinated dibenzodioxins: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
[[Image:Dioxin-3D-vdW.png|right|220px|thumb|[[Space-filling model]] of 2,3,7,8- tetrachlorodibenzo-''p''-dioxin]] |
[[Image:Dioxin-3D-vdW.png|right|220px|thumb|[[Space-filling model]] of 2,3,7,8- tetrachlorodibenzo-''p''-dioxin]] |
||
[[Image:Dioxin-2D-skeletal.svg|right|220px|thumb|Structure of [[2,3,7,8-tetrachlorodibenzo-p-dioxin]] (TCDD)]] |
[[Image:Dioxin-2D-skeletal.svg|right|220px|thumb|Structure of [[2,3,7,8-tetrachlorodibenzo-p-dioxin]] (TCDD)]] |
||
'''Dioxin''' is the popular name for the family of [[organohalogen|halogenated organic compounds]], the most common consisting of [[chlorine|polychlorinated]] [[benzene|dibenzo]][[furan]]s (PCDFs) and polychlorinated dibenzodioxins (PCDDs). PCDD/PCDFs (PCDD/Fs) have been shown to [[bioaccumulate]] in humans and [[wildlife]] due to their [[lipophilic]] properties, and are known [[teratogens]], [[mutagens]], and suspected human [[carcinogens]]. Recently, polybrominated dibenzofurans and dibenzodioxins have been discovered as impurities in brominated [[flame retardant]]s, such as [[PBDE|polybrominated diphenyl ethers]]. |
'''Dioxin''' is the popular name for the family of [[organohalogen|halogenated organic compounds]], the most common consisting of [[chlorine|polychlorinated]] [[benzene|dibenzo]][[furan]]s (PCDFs) and polychlorinated dibenzodioxins (PCDDs). PCDD/PCDFs (PCDD/Fs) have been shown to [[bioaccumulate]] in humans and [[wildlife]] due to their [[lipophilic]] properties, and are known [[teratogens]], [[mutagens]], and suspected human [[carcinogens]]. Recently, polybrominated dibenzofurans and dibenzodioxins have been discovered as impurities in brominated [[flame retardant]]s, such as [[PBDE|polybrominated diphenyl ethers]]. |
||
== Chemical structure == |
== Chemical structure == |
||
[[Image:Dibenzo-p-dioxin-numbering-2D-skeletal.png|thumb|right|200px|The [[skeletal formula]] and [[substituent]] numbering scheme of the parent compound dibenzo-''p''-dioxin]] |
[[Image:Dibenzo-p-dioxin-numbering-2D-skeletal.png|thumb|right|200px|The [[skeletal formula]] and [[substituent]] numbering scheme of the parent compound dibenzo-''p''-dioxin]] |
||
The basic structure of PCDD/Fs comprises two [[benzene ring]]s joined by either a single ([[furan]]) or a double oxygen bridge (dioxin). Although the first synthesis goes back to the year 1872, the structure of 2,3,7,8-tetrachlorodibenzo-''p''-dioxin was unknown until 1957.<ref>{{cite journal |
The basic structure of PCDD/Fs comprises two [[benzene ring]]s joined by either a single ([[furan]]) or a double oxygen bridge (dioxin). Although the first synthesis goes back to the year 1872, the structure of 2,3,7,8-tetrachlorodibenzo-''p''-dioxin was unknown until 1957.<ref> |
||
{{cite journal |
|||
|author= Sandermann, W., Stockmann, H., Casten, R. |
|author= Sandermann, W., Stockmann, H., Casten, R. |
||
|title = Über die Pyrolyse des Pentachlorphenols (Pyrolysis of pentachlorophenol) |
|title = Über die Pyrolyse des Pentachlorphenols (Pyrolysis of pentachlorophenol) |
||
| Line 14: | Line 17: | ||
| pages = 690—692 |
| pages = 690—692 |
||
| doi =10.1002/cber.19570900506}} |
| doi =10.1002/cber.19570900506}} |
||
</ref> Chlorine atoms are attached to the basic structure at any of 8 different places on the molecule, positions 1–4 and 6–9. There are 210 different types of PCDD/F [[congener]]s (herein, a congener means a related dioxin compound) comprising of 75 PCDDs and 135 PCDFs. The toxicity of PCDD/Fs depends on the number and position of the chlorine atoms; only congeners that have chlorines in the 2, 3, 7, and 8 positions have any observable toxicity. Out of the 210 PCDD/F compounds in total, only 17 congeners (7 PCDDs and 10 PCDFs) have chlorine atoms in the relevant positions to be considered toxic by the [[NATO]] ''Committee on the Challenges to Modern Society'' (NATO/CCMS) international toxic equivalent (I-TEQ) scheme. |
</ref> Chlorine atoms are attached to the basic structure at any of 8 different places on the molecule, positions 1–4 and 6–9. There are 210 different types of PCDD/F [[congener]]s (herein, a congener means a related dioxin compound) comprising of 75 PCDDs and 135 PCDFs. The toxicity of PCDD/Fs depends on the number and position of the chlorine atoms; only congeners that have chlorines in the 2, 3, 7, and 8 positions have any observable toxicity. Out of the 210 PCDD/F compounds in total, only 17 congeners (7 PCDDs and 10 PCDFs) have chlorine atoms in the relevant positions to be considered toxic by the [[NATO]] ''Committee on the Challenges to Modern Society'' (NATO/CCMS) international toxic equivalent (I-TEQ) scheme. |
||
== Historical perspective == |
== Historical perspective == |
||
Dioxins didn’t exist in nature prior to [[industrialization]] except in very trace amounts due to natural combustion and geological processes<ref>http://ec.europa.eu/environment/dioxin/pdf/task6.pdf </ref> <ref>http://www.cfsan.fda.gov/~lrd/dioxinqa.html#g9</ref>. The first synthesis of dioxin dates back to 1872. Today they are found in all humans, with higher levels commonly found in persons living in more industrialized countries. The most toxic dioxin, '''2,3,7,8 tetrachlorodibenzo-''p''-dioxin''' (TCDD), became well known as a contaminant of [[Agent Orange]] herbicide used in the Vietnam War<ref> |
|||
Dioxins did not exist in nature prior to [[industrialization]], except in very trace amounts due to natural combustion and geological processes<ref> |
|||
{{cite web |
|||
| last = |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Compilation of EU Dioxin Exposure and Health Data |
|||
| work = European Commission DG Environment |
|||
| publisher = |
|||
| date = 1999 |
|||
| url = http://ec.europa.eu/environment/dioxin/pdf/task6.pdf |
|||
| format = .pdf |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref><ref> |
|||
{{cite web |
|||
| last = |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Questions and Answers About Dioxins |
|||
| work = |
|||
| publisher = |
|||
| date = |
|||
| url = http://www.cfsan.fda.gov/~lrd/dioxinqa.html#g9 |
|||
| format = |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref>. The first synthesis of dioxin dates back to 1872. Today they are found in all humans, with higher levels commonly found in persons living in more industrialized countries. The most toxic dioxin, '''2,3,7,8 tetrachlorodibenzo-''p''-dioxin''' (TCDD), became well known as a contaminant of [[Agent Orange]], an herbicide used in the Vietnam War<ref> |
|||
{{cite journal |
{{cite journal |
||
| author = Schecter A. Birnbaum L., Ryan J. J., Constable J. D. |
| author = Schecter A. Birnbaum L., Ryan J. J., Constable J. D. |
||
| Line 28: | Line 63: | ||
| doi = 10.1016/j.envres.2005.12.003 |
| doi = 10.1016/j.envres.2005.12.003 |
||
}} |
}} |
||
</ref>. Later dioxins were found in Times Beach, Missouri<ref> |
|||
</ref>. Later dioxins were found in Times Beach, Missouri<ref>http://www.epa.gov/history/topics/times/03.htm</ref> and Love Canal, New York of USA<ref>http://www.epa.gov/history/topics/lovecanal/04.htm</ref> and Seveso, Italy<ref>http://www.unu.edu/unupress/unupbooks/uu21le/uu21le09.htm</ref>. Dioxins have already been in the news with poisoning of President Viktor [[Yushchenko]] of [[Ukraine]] in 2004. |
|||
{{cite web |
|||
| last = |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Times Beach Record of Decision Signed |
|||
| work = |
|||
| publisher = EPA |
|||
| date = September 30, 1998 |
|||
| url = http://www.epa.gov/history/topics/times/03.htm |
|||
| format = |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref> and Love Canal, New York of USA<ref> |
|||
{{cite web |
|||
| last = |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Love Canal Record of Decision Signed |
|||
| work = |
|||
| publisher = EPA |
|||
| date = October 26, 1987 |
|||
| url = http://www.epa.gov/history/topics/lovecanal/04.htm |
|||
| format = |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref> and Seveso, Italy<ref> |
|||
{{cite web |
|||
| last = De Marchi, B., et al. |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Seveso, A Paradoxical Classical Disaster |
|||
| work = |
|||
| publisher = |
|||
| date = |
|||
| url = http://www.unu.edu/unupress/unupbooks/uu21le/uu21le09.htm |
|||
| format = |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref>. Dioxins have already been in the news with poisoning of President Viktor [[Yushchenko]] of [[Ukraine]] in 2004. |
|||
== Toxicity == |
== Toxicity == |
||
Dioxins are carcinogenic in higher amounts |
Dioxins are carcinogenic in higher amounts and cause developmental and reproductive problems. They are absorbed primarily through the dietary intake of fat, as this is where they accumulate in animals and humans. In humans, the highly chlorinated dioxins are stored in fatty tissues and are neither readily metabolized nor excreted. This persistence is the consequence of their structure. In contrast, dioxins with few chlorines always contain hydrogen atoms on the adjacent pairs of carbons at which hydroxyl groups can readily be added in the biochemical reactions and tend to be excreted rather than stored for long time. |
||
2,3,7,8-tetrachlorodibenzo-''p''-dioxin (TCDD) is the most toxic of the [[congener]]s. Other dioxin congeners (or mixtures thereof) are given a toxicity rating from 0 to 1, where TCDD = 1. This toxicity rating is called the Toxic Equivalence Factor, or TEF. TEFs are consensus values and, because of the strong species dependence for toxicity, are listed separately for mammals, fish and birds. TEFs for mammalian species are generally applicable to human risk calculations. The TEFs have been developed from detailed assessment of literature data to facilitate both risk assessment and regulatory control.<ref> |
2,3,7,8-tetrachlorodibenzo-''p''-dioxin (TCDD) is the most toxic of the [[congener]]s. Other dioxin congeners (or mixtures thereof) are given a toxicity rating from 0 to 1, where TCDD = 1. This toxicity rating is called the Toxic Equivalence Factor, or TEF. TEFs are consensus values and, because of the strong species dependence for toxicity, are listed separately for mammals, fish and birds. TEFs for mammalian species are generally applicable to human risk calculations. The TEFs have been developed from detailed assessment of literature data to facilitate both risk assessment and regulatory control.<ref> |
||
{{cite journal |
{{cite journal |
||
|author= M. Van den Berg, L. S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, Martin Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker, Richard E. Peterson |
|author= M. Van den Berg, L. S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, Martin Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker, Richard E. Peterson |
||
| Line 43: | Line 126: | ||
|pages = 223–241 |
|pages = 223–241 |
||
| doi =10.1093/toxsci/kfl055 |
| doi =10.1093/toxsci/kfl055 |
||
| id = PIM 16829543}} </ref> Many other compounds may also have dioxin-like properties, particularly non-ortho [[PCBs]], some of which can have TEFs as high as 0.1. |
| id = PIM 16829543 |
||
}} |
|||
</ref> Many other compounds may also have dioxin-like properties, particularly non-ortho [[PCBs]], some of which can have TEFs as high as 0.1. |
|||
The total dioxin toxic equivalency (TEQ) value expresses the toxicity as if the mixture were pure TCDD. The TEQ approach and current TEFs have been adopted internationally as the most appropriate way to estimate the potential health risks of mixture of dioxins. |
The total dioxin toxic equivalency (TEQ) value expresses the toxicity as if the mixture were pure TCDD. The TEQ approach and current TEFs have been adopted internationally as the most appropriate way to estimate the potential health risks of mixture of dioxins. |
||
| Line 51: | Line 136: | ||
==Sources of dioxin== |
==Sources of dioxin== |
||
The [[United States]] [[Environmental Protection Agency]] [[Dioxin Reassessment Report]] is possibly the most comprehensive review of dioxin, but other countries now have substantial research. [[Australia]], [[New Zealand]] and the [[United Kingdom]] all have substantial research into [[body burden]]s and sources. Tolerable daily, monthly or annual intakes have been set by the [[World Health Organization]] and a number of governments. Dioxin enters the general population almost exclusively from ingestion of food, specifically through the consumption of fish, meat, and dairy products since dioxins are fat-soluble and readily climb the [[food chain]].< |
The [[United States]] [[Environmental Protection Agency]] [[Dioxin Reassessment Report]] is possibly the most comprehensive review of dioxin, but other countries now have substantial research. [[Australia]], [[New Zealand]] and the [[United Kingdom]] all have substantial research into [[body burden]]s and sources. Tolerable daily, monthly or annual intakes have been set by the [[World Health Organization]] and a number of governments. Dioxin enters the general population almost exclusively from ingestion of food, specifically through the consumption of fish, meat, and dairy products since dioxins are fat-soluble and readily climb the [[food chain]].<ref> |
||
{{cite journal |
|||
|author= Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, Silver A, Schmitz M. |
|||
|title = Intake of dioxins and related compounds from food in the U.S. population. |
|||
| journal = Journal of Toxicology and Environmental Health, Part A |
|||
| year = 2001 |
|||
|volume = 11 |
|||
|pages = 1-18 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref> |
|||
Occupational exposure is an issue for some in the chemical industry, or in the application of chemicals, notably [[herbicide]]s. Inhalation has been a problem for people living near substantial point sources where emissions are not adequately controlled. In many developed nations there are now emissions regulations which have alleviated some concerns, although the lack of constant sampling of dioxin emissions causes concern about the understatement of emissions. In [[Belgium]], through the introduction of a process called [[AMESA]], constant sampling showed that periodic sampling understated emissions by a factor of 30 to 50 times. Few facilities have constant sampling. |
Occupational exposure is an issue for some in the chemical industry, or in the application of chemicals, notably [[herbicide]]s. Inhalation has been a problem for people living near substantial point sources where emissions are not adequately controlled. In many developed nations there are now emissions regulations which have alleviated some concerns, although the lack of constant sampling of dioxin emissions causes concern about the understatement of emissions. In [[Belgium]], through the introduction of a process called [[AMESA]], constant sampling showed that periodic sampling understated emissions by a factor of 30 to 50 times. Few facilities have constant sampling. |
||
| Line 73: | Line 170: | ||
In incineration, dioxins can also reform in the [[Earth's atmosphere|atmosphere]] above the stack as the exhaust gases cool through a temperature window of 600 to 200°C. The most common method of reducing dioxins reforming or forming de novo is through rapid (30 millisecond) quenching of the exhaust gases through that 400°C window.<ref> |
In incineration, dioxins can also reform in the [[Earth's atmosphere|atmosphere]] above the stack as the exhaust gases cool through a temperature window of 600 to 200°C. The most common method of reducing dioxins reforming or forming de novo is through rapid (30 millisecond) quenching of the exhaust gases through that 400°C window.<ref> |
||
{{cite journal |
{{cite journal |
||
| author = Gordon McKay |
| author = Gordon McKay |
||
| Line 86: | Line 184: | ||
[[Image:Dioxin_chart.png|thumb|400px|right|A chart illustrating how much dioxin the average American consumes per day.]] |
[[Image:Dioxin_chart.png|thumb|400px|right|A chart illustrating how much dioxin the average American consumes per day.]] |
||
Dioxins are also generated in reactions that do not involve burning — such as bleaching fibers for paper or textiles, and in the manufacture of chlorinated phenols, particularly when reaction temperature is not well controlled. Affected compounds include the wood preservative [[pentachlorophenol]], and also [[herbicide]]s such as [[2,4-D|2,4-dichlorophenoxyacetic acid]] (or 2,4-D) and [[2,4,5-trichlorophenoxyacetic acid]] (2,4,5-T). Higher levels of chlorination require higher reaction temperatures and greater dioxin production. See [[Agent Orange]] for more on contamination problems in the [[1960s]]. Dioxins may also be formed during the [[photochemical]] breakdown of the common antimicrobial compound [[triclosan]].<ref>{{cite journal|author=Latch D. E., Packer J. L., Stender B. L., VanOverbeke J., Arnold W. A., McNeill K.|title=Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products|journal=Environmental toxicology and chemistry|year= 2005|volume=24|issue=3|pages=517–525| |
Dioxins are also generated in reactions that do not involve burning — such as bleaching fibers for paper or textiles, and in the manufacture of chlorinated phenols, particularly when reaction temperature is not well controlled. Affected compounds include the wood preservative [[pentachlorophenol]], and also [[herbicide]]s such as [[2,4-D|2,4-dichlorophenoxyacetic acid]] (or 2,4-D) and [[2,4,5-trichlorophenoxyacetic acid]] (2,4,5-T). Higher levels of chlorination require higher reaction temperatures and greater dioxin production. See [[Agent Orange]] for more on contamination problems in the [[1960s]]. Dioxins may also be formed during the [[photochemical]] breakdown of the common antimicrobial compound [[triclosan]].<ref> |
||
{{cite journal |
|||
|author=Latch D. E., Packer J. L., Stender B. L., VanOverbeke J., Arnold W. A., McNeill K. |
|||
|title=Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products |
|||
|journal=Environmental toxicology and chemistry |
|||
|year= 2005 |
|||
|volume=24 |
|||
|issue=3 |
|||
|pages=517–525| |
|||
doi= 10.1897/04-243R.1}} |
|||
</ref> |
|||
Dioxins are present in minuscule amounts in a wide range of materials used by humans — including practically all substances manufactured using [[plastic]]s, [[resin]]s or [[bleach]]es.{{Fact|date=April 2007}} Such materials include [[tampon]]s, and a wide variety of food packaging substances{{Fact|date=April 2007}}. The use of these materials means that all Western humans receive at least a ''very'' small daily dose of dioxin—however, it is disputed whether such exceptionally tiny exposures have any clinical relevance. It is even controversially discussed if dioxins might have a non-linear dose-response curve with beneficial health effects in a certain lower dose range, a phenomenon called [[hormesis]]. |
Dioxins are present in minuscule amounts in a wide range of materials used by humans — including practically all substances manufactured using [[plastic]]s, [[resin]]s or [[bleach]]es.{{Fact|date=April 2007}} Such materials include [[tampon]]s, and a wide variety of food packaging substances{{Fact|date=April 2007}}. The use of these materials means that all Western humans receive at least a ''very'' small daily dose of dioxin—however, it is disputed whether such exceptionally tiny exposures have any clinical relevance. It is even controversially discussed if dioxins might have a non-linear dose-response curve with beneficial health effects in a certain lower dose range, a phenomenon called [[hormesis]]. |
||
Dietary sources of dioxin in the United States have been analyzed by the [[EPA]] and other scientists. Summaries of the primary dietary sources are in the following two graphics (note pg = picogram, or one trillionth of a gram, or 10<sup>−12</sup>, or 0.000000000001 g).< |
Dietary sources of dioxin in the United States have been analyzed by the [[EPA]] and other scientists. Summaries of the primary dietary sources are in the following two graphics (note pg = picogram, or one trillionth of a gram, or 10<sup>−12</sup>, or 0.000000000001 g).<ref> |
||
{{cite journal |
|||
|author= Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, Silver A, Schmitz M. |
|||
|title = Intake of dioxins and related compounds from food in the U.S. population. |
|||
| journal = Journal of Toxicology and Environmental Health, Part A |
|||
| year = 2001 |
|||
|volume = 11 |
|||
|pages = 1-18 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref> |
|||
== Health effects in humans == |
== Health effects in humans == |
||
Dioxins build up primarily in fatty tissues over time ([[bioaccumulate]]), so even small exposures may eventually reach dangerous levels. In 1994, EPA reported that dioxin is a probable carcinogen, but notes that non-cancer effects (reproduction and sexual development, immune system) may pose an even greater threat to human health. [[TCDD]], the most toxic of the dibenzodioxins, has a half-life of approximately 7 years in humans, but at high concentrations, the elimination rate is enhanced by metabolism.<ref> |
Dioxins build up primarily in fatty tissues over time ([[bioaccumulate]]), so even small exposures may eventually reach dangerous levels. In 1994, EPA reported that dioxin is a probable carcinogen, but notes that non-cancer effects (reproduction and sexual development, immune system) may pose an even greater threat to human health. [[TCDD]], the most toxic of the dibenzodioxins, has a half-life of approximately 7 years in humans, but at high concentrations, the elimination rate is enhanced by metabolism.<ref> |
||
{{cite journal |
|||
|author= Geusau A, Schmaldienst S, Derfler K, Papke O, Abraham K. |
|||
|title = Severe 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) intoxication: kinetics and trials to enhance elimination in two patients. |
|||
| journal = Arch Toxicol. |
|||
| year = 2002 |
|||
|volume = 76 |
|||
|issue = 5-6 |
|||
|pages = 316-325 |
|||
| doi = 10.1007/s00204-002-0345-7 |
|||
| id = |
|||
}} |
|||
.</ref> The health effects of dioxins are mediated by their action on a cellular receptor, the Aryl Hydrocarbon Receptor (AhR).<ref> |
|||
{{cite journal |
|||
|author= Mimura J, Fujii-Kuriyama Y |
|||
|title = Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. |
|||
| journal = Biochem Pharmacol |
|||
| year = 2006 |
|||
|volume = 72 |
|||
|issue = 4 |
|||
|pages = 393-404 |
|||
| doi = 10.1016/j.bcp.2006.01.017 |
|||
| id = |
|||
}} |
|||
</ref> |
|||
Dioxins also accumulate in food chains in a fashion similar to other chlorinated compounds ([[bioaccumulate]]). This means that even small concentrations in contaminated water can be concentrated up a food chain to dangerous levels due to the long biological half life and low solubility of dioxins. |
Dioxins also accumulate in food chains in a fashion similar to other chlorinated compounds ([[bioaccumulate]]). This means that even small concentrations in contaminated water can be concentrated up a food chain to dangerous levels due to the long biological half life and low solubility of dioxins. |
||
| Line 99: | Line 248: | ||
Exposure to high levels of dioxin in humans causes a severe form of persistent [[Acne vulgaris|acne]], known as [[chloracne]] [http://www.ehponline.org/members/2001/109p865-869geusau/geusau-full.html] and [[hyperpigmentation]]. Other effects in humans may include: |
Exposure to high levels of dioxin in humans causes a severe form of persistent [[Acne vulgaris|acne]], known as [[chloracne]] [http://www.ehponline.org/members/2001/109p865-869geusau/geusau-full.html] and [[hyperpigmentation]]. Other effects in humans may include: |
||
* Developmental abnormalities in the [[tooth enamel|enamel]] of children's [[teeth]]. |
|||
* Developmental abnormalities in the [[tooth enamel|enamel]] of children's [[teeth]].<ref>http://ehp.niehs.nih.gov/docs/2004/6920/abstract.html</ref><ref>{{cite journal|title = Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons|journal = Critical Review in Toxicology | year =1993| volume =23|issue 3|pages=283–335|author= Peterson R. E., Theobald H. M., Kimmel G. L.}}</ref> |
|||
<ref> |
|||
{{cite journal |
|||
|author = Satu Alaluusua, Pier Calderara, Pier Mario Gerthoux, Pirjo-Liisa Lukinmaa, Outi Kovero, Larry Needham, Donald G. Patterson Jr., Jouko Tuomisto, and Paolo Mocarelli |
|||
|title = Developmental Dental Aberrations After the Dioxin Accident in Seveso |
|||
| journal = Environmental Health Perspectives |
|||
| year = 2004 |
|||
|volume = 112 |
|||
|issue = 13 |
|||
|pages = 1313-1318 |
|||
| doi = 10.1289/ehp.6920 |
|||
| id = |
|||
}} |
|||
</ref> |
|||
<ref> |
|||
{{cite journal |
|||
|title = Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons |
|||
|journal = Critical Review in Toxicology |
|||
| year =1993 |
|||
| volume =23 |
|||
|issue 3 |
|||
|pages=283–335 |
|||
|author= Peterson R. E., Theobald H. M., Kimmel G. L.}} |
|||
</ref> |
|||
* Central and Peripheral Nervous System pathology{{Fact|date=February 2007}} |
* Central and Peripheral Nervous System pathology{{Fact|date=February 2007}} |
||
| Line 108: | Line 280: | ||
== Health effects in other animals == |
== Health effects in other animals == |
||
While it has been difficult to prove that dioxins cause specific health effects in humans due to the lack of controlled dose experiments, studies in animals have shown that dioxin causes a wide variety of toxic effects. In particular, TCDD has been shown to be [[teratogenic]], [[mutagenic]], [[carcinogenic]], [[immunotoxic]], and [[hepatotoxic]]. Furthermore, alterations in multiple [[endocrine]] and [[growth factor]] systems have been reported. The most sensitive effects, observed in multiple species, appear to be developmental, including effects on the developing [[immune]], [[nervous]], and [[reproductive]] systems <ref>Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. |
While it has been difficult to prove that dioxins cause specific health effects in humans due to the lack of controlled dose experiments, studies in animals have shown that dioxin causes a wide variety of toxic effects. In particular, TCDD has been shown to be [[teratogenic]], [[mutagenic]], [[carcinogenic]], [[immunotoxic]], and [[hepatotoxic]]. Furthermore, alterations in multiple [[endocrine]] and [[growth factor]] systems have been reported. The most sensitive effects, observed in multiple species, appear to be developmental, including effects on the developing [[immune]], [[nervous]], and [[reproductive]] systems <ref> |
||
{{cite journal |
|||
|author = Birnbaum LS, Tuomisto J. |
|||
|title = Non-carcinogenic effects of TCDD in animals. |
|||
| journal = Food Addit Contam. |
|||
| year = 2000 |
|||
|volume = 17 |
|||
|issue = 4 |
|||
|pages = 275-288 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref>. These effects are caused at [[body_burden|body burdens]] close to those reported in humans. |
|||
Among the animals for which TCDD toxicity has been studied, there is strong evidence for the following effects: |
Among the animals for which TCDD toxicity has been studied, there is strong evidence for the following effects: |
||
* Birth defects ([[teratogenic|teratogenicity]]) |
* Birth defects ([[teratogenic|teratogenicity]]) |
||
:In rodents, including rats <ref> |
|||
:In rodents, including rats <ref> National Toxicology Program. Tech Rep Ser. 2006 Apr;(521):4-232.</ref>, mice <ref> Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott |
|||
BD. Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicol Sci. 1999 Jan;47(1):86-92.</ref>, hamsters and guinea pigs <ref>{{cite journal |
|||
{{cite journal |
|||
|author = NTP |
|||
|title = NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies). |
|||
| journal = Natl Toxicol Program Tech Rep Ser. |
|||
| year = 2006 |
|||
|volume = 521 |
|||
|issue = |
|||
|pages = |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref>, mice <ref> |
|||
{{cite journal |
|||
|author = Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott |
|||
BD. |
|||
|title = Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. |
|||
| journal = Toxicological Sciences |
|||
| year = 1999 |
|||
|volume = 47 |
|||
|issue = 1 |
|||
|pages = 86-92 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
.</ref>, hamsters and guinea pigs <ref> |
|||
{{cite journal |
|||
| author = Kransler KM, McGarrigle BP, Olson JR. |
| author = Kransler KM, McGarrigle BP, Olson JR. |
||
| year = 2007 |
| year = 2007 |
||
| Line 124: | Line 336: | ||
| doi = 10.1016/j.tox.2006.10.019 |
| doi = 10.1016/j.tox.2006.10.019 |
||
}} |
}} |
||
</ref>; birds <ref> |
|||
</ref>; birds <ref> Bruggeman V, Swennen Q, De Ketelaere B, Onagbesan O, Tona K, Decuypere E. Embryonic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chickens: effects of dose and embryonic stage on hatchability and growth.Comp Biochem Physiol C Toxicol Pharmacol. 2003 Sep;136(1):17-28.</ref>; and fish <ref> Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth Defects Res A Clin Mol Teratol. 2006 Jan;76(1):7-18.</ref>. |
|||
{{cite journal |
|||
| author = Bruggeman V, Swennen Q, De Ketelaere B, Onagbesan O, Tona K, Decuypere E. |
|||
| year = 2003 |
|||
| title = Embryonic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chickens: effects of dose and embryonic stage on hatchability and growth. |
|||
| journal = Comp Biochem Physiol C Toxicol Pharmacol. |
|||
| volume = 136 |
|||
| issue = 1 |
|||
| pages = 17-28 |
|||
| doi = 10.1016/S1532-0456(03)00168-6 |
|||
}} |
|||
.</ref>; and fish <ref> |
|||
{{cite journal |
|||
| author = Carney SA, Prasch AL, Heideman W, Peterson RE. |
|||
| year = 2006 |
|||
| title = Understanding dioxin developmental toxicity using the zebrafish model. |
|||
| journal = Birth Defects Res A Clin Mol Teratol. |
|||
| volume = 76 |
|||
| issue = 1 |
|||
| pages = 7-18 |
|||
| doi = 10.1002/bdra.20216 |
|||
}} |
|||
</ref>. |
|||
* Cancer (including [[neoplasms]] in the mammalian lung, oral/nasal cavities, [[thyroid]] and [[adrenal]] glands, and liver, [[squamous cell]] carcinoma, and various animal [[hepatocellular carcinoma|hepatocarcinomas]]) |
* Cancer (including [[neoplasms]] in the mammalian lung, oral/nasal cavities, [[thyroid]] and [[adrenal]] glands, and liver, [[squamous cell]] carcinoma, and various animal [[hepatocellular carcinoma|hepatocarcinomas]]) |
||
:In rodents <ref> |
|||
:In rodents <ref> National Toxicology Program. Tech Rep Ser. 2006 Apr;(521):4-232.</ref><ref>Selected lesions of dioxin in laboratory rodents. Toxicol Pathol. 1997 Jan-Feb;25(1):72-9.</ref><ref> Huff JE, Salmon AG, Hooper NK, Zeise L. Long-term carcinogenesis studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin and hexachlorodibenzo-p-dioxins. Cell Biol Toxicol. 1991 Jan;7(1):67-94.</ref> and fish <ref>Grinwis GC, Vethaak AD, Wester PW, Vos JG. Toxicol Lett. 2000 Mar 15;112-113:289-301. </ref> |
|||
{{cite journal |
|||
|author = NTP |
|||
|title = NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies). |
|||
| journal = Natl Toxicol Program Tech Rep Ser. |
|||
| year = 2006 |
|||
|volume = 521 |
|||
|issue = |
|||
|pages = |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref><ref> |
|||
{{cite journal |
|||
|author = NTP |
|||
|title = Selected lesions of dioxin in laboratory rodents. |
|||
| journal = Toxicol Pathol. |
|||
| year = 1997 |
|||
|volume = 25 |
|||
|issue = 1 |
|||
|pages = 72-79 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref> and fish<ref> |
|||
{{cite journal |
|||
|author = Grinwis GC, Vethaak AD, Wester PW, Vos JG. |
|||
|title = Toxicology of environmental chemicals in the flounder (Platichthys flesus) with emphasis on the immune system: field, semi-field (mesocosm) and laboratory studies. |
|||
| journal = Toxicol Lett. |
|||
| year = 2000 |
|||
|volume = 112-113 |
|||
|issue = |
|||
|pages = 289-301 |
|||
| doi = 10.1016/S0378-4274(99)00239-8 |
|||
| id = |
|||
}} |
|||
</ref> |
|||
* Hepatotoxicity (liver toxicity) |
* Hepatotoxicity (liver toxicity) |
||
:In rodents <ref> |
|||
:In rodents <ref>Selected lesions of dioxin in laboratory rodents. Toxicol Pathol. 1997 Jan-Feb;25(1):72-9.</ref>; chickens <ref>El-Sabeawy F, Enan E, Lasley B. Biochemical and toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in immature |
|||
{{cite journal |
|||
male and female chickens. Comp Biochem Physiol C Toxicol Pharmacol. 2001 Aug;129(4):317-27.</ref>; and fish <ref>Zodrow JM, Stegeman JJ, Tanguay RL. Histological analysis of acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD) in zebrafish. Aquat Toxicol. 2004 Jan 7;66(1):25-38.</ref> |
|||
|author = NTP |
|||
|title = Selected lesions of dioxin in laboratory rodents. |
|||
| journal = Toxicol Pathol. |
|||
| year = 1997 |
|||
|volume = 25 |
|||
|issue = 1 |
|||
|pages = 72-79 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref>; chickens <ref> |
|||
{{cite journal |
|||
|author = El-Sabeawy F, Enan E, Lasley B. |
|||
|title = Biochemical and toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in immature |
|||
male and female chickens. |
|||
| journal = Comp Biochem Physiol C Toxicol Pharmacol. |
|||
| year = 2001 |
|||
|volume = 129 |
|||
|issue = 4 |
|||
|pages = 317-327 |
|||
| doi = 10.1016/S1532-0456(01)00199-5 |
|||
| id = |
|||
}} |
|||
</ref>; and fish <ref> |
|||
{{cite journal |
|||
|author = Zodrow JM, Stegeman JJ, Tanguay RL. |
|||
|title = Histological analysis of acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD) in zebrafish. |
|||
| journal = Aquat Toxicol. |
|||
| year = 2004 |
|||
|volume = 66 |
|||
|issue = 1 |
|||
|pages = 25-38 |
|||
| doi = 10.1016/j.aquatox.2003.07.002 |
|||
| id = |
|||
}} |
|||
.</ref> |
|||
*Endocrine disruption |
*Endocrine disruption |
||
:In rodents{{Fact|date=April 2007}} and fish <ref> Heiden TK, Carvan MJ 3rd, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum |
:In rodents{{Fact|date=April 2007}} and fish <ref> |
||
{{cite journal |
|||
|author = Heiden TK, Carvan MJ 3rd, Hutz RJ. |
|||
|title = Inhibition of follicular development, vitellogenesis, and serum |
|||
17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. |
17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. |
||
| journal = Toxicological Sciences |
|||
| year = 2006 |
|||
|volume = 90 |
|||
|issue = 2 |
|||
|pages = 490-499 |
|||
| doi = 10.1093/toxsci/kfj085 |
|||
| id = |
|||
}} |
|||
</ref> |
|||
*Immunosuppression |
*Immunosuppression |
||
:In rodents{{Fact|date=April 2007}} and fish{{Fact|date=April 2007}}. |
:In rodents{{Fact|date=April 2007}} and fish{{Fact|date=April 2007}}. |
||
*Learning <ref> |
|||
*Learning <ref> Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. 2000 Apr;17(4):275-88. </ref> |
|||
{{cite journal |
|||
|author = Birnbaum LS, Tuomisto J. |
|||
|title = Non-carcinogenic effects of TCDD in animals. |
|||
| journal = Food Addit Contam. |
|||
| year = 2000 |
|||
|volume = 17 |
|||
|issue = 4 |
|||
|pages = 275-288 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref> |
|||
== Studies of dioxin's effects in Vietnam == |
== Studies of dioxin's effects in Vietnam == |
||
[[United States|US]] veterans' groups and [[Vietnam]]ese groups, including the Vietnamese government, have convened scientific studies to explore their belief that dioxins were responsible for a host of disorders, including tens of thousands of birth defects in children, amongst Vietnam veterans as well as an estimated one million Vietnamese, through their exposure to [[Agent Orange]] during the [[Vietnam War]], which was found to be highly contaminated with TCDD. Several exposure studies showed that some US Vietnam Veterans who were exposed to Agent Orange had serum TCDD levels up to 600 ppt in many years after they left Vietnam, compared to general population levels of approximately 1 to 2 ppt of TCDD. In Vietnam TCDD levels up to 1,000,000 ppt have been found in soil or sediments from Agent Orange contaminated areas 3 to 4 decades after spraying. In addition, elevated levels have been measured in food and wildlife in Vietnam<ref>Olie, K., Schecter, A., et al., |
[[United States|US]] veterans' groups and [[Vietnam]]ese groups, including the Vietnamese government, have convened scientific studies to explore their belief that dioxins were responsible for a host of disorders, including tens of thousands of birth defects in children, amongst Vietnam veterans as well as an estimated one million Vietnamese, through their exposure to [[Agent Orange]] during the [[Vietnam War]], which was found to be highly contaminated with TCDD. Several exposure studies showed that some US Vietnam Veterans who were exposed to Agent Orange had serum TCDD levels up to 600 ppt in many years after they left Vietnam, compared to general population levels of approximately 1 to 2 ppt of TCDD. In Vietnam TCDD levels up to 1,000,000 ppt have been found in soil or sediments from Agent Orange contaminated areas 3 to 4 decades after spraying. In addition, elevated levels have been measured in food and wildlife in Vietnam<ref> |
||
{{cite journal |
|||
|author = Olie, K., Schecter, A., et al., |
|||
|title = Chlorinated dioxin and dibenzofuran levels in food and wildlife samples in the North and South of Vietnam |
|||
| journal = Chemosphere |
|||
| year = 1989 |
|||
|volume = 19 |
|||
|issue = |
|||
|pages = 493-496 |
|||
| doi = |
|||
| id = |
|||
}} |
|||
</ref> |
|||
The most recent study, paid for by the [[National Academy of Sciences]], was released in an April [[2003]] report. This report is currently (March 2007) being revised for release again later in 2007. |
The most recent study, paid for by the [[National Academy of Sciences]], was released in an April [[2003]] report. This report is currently (March 2007) being revised for release again later in 2007. |
||
The [[Center for Disease Control]] found that dioxin levels in Vietnam veterans<ref> |
|||
The [[Center for Disease Control]] found that dioxin levels in Vietnam veterans<ref>http://www.cdc.gov/nceh/veterans/vet_hlth_actvy.pdf</ref> were in no way atypical when compared against the rest of the population. The only exception existed for those who directly handled Agent Orange. These were members of [[Operation Ranch Hand]]. Long term studies of the members of Ranch Hand have thus far uncovered a possibility of elevated risks of diabetes. |
|||
{{cite web |
|||
| last = CDC |
|||
| first = |
|||
| authorlink = |
|||
| coauthors = |
|||
| title = Veteran’s Health Activities |
|||
| work = |
|||
| publisher = |
|||
| date = |
|||
| url = http://www.cdc.gov/nceh/veterans/vet_hlth_actvy.pdf |
|||
| format = .pdf |
|||
| doi = |
|||
| accessdate = |
|||
}} |
|||
</ref> were in no way atypical when compared against the rest of the population. The only exception existed for those who directly handled Agent Orange. These were members of [[Operation Ranch Hand]]. Long term studies of the members of Ranch Hand have thus far uncovered a possibility of elevated risks of diabetes. |
|||
== Dioxin exposure incidents == |
== Dioxin exposure incidents == |
||
Revision as of 00:55, 26 April 2007


Dioxin is the popular name for the family of halogenated organic compounds, the most common consisting of polychlorinated dibenzofurans (PCDFs) and polychlorinated dibenzodioxins (PCDDs). PCDD/PCDFs (PCDD/Fs) have been shown to bioaccumulate in humans and wildlife due to their lipophilic properties, and are known teratogens, mutagens, and suspected human carcinogens. Recently, polybrominated dibenzofurans and dibenzodioxins have been discovered as impurities in brominated flame retardants, such as polybrominated diphenyl ethers.
Chemical structure

The basic structure of PCDD/Fs comprises two benzene rings joined by either a single (furan) or a double oxygen bridge (dioxin). Although the first synthesis goes back to the year 1872, the structure of 2,3,7,8-tetrachlorodibenzo-p-dioxin was unknown until 1957.[1] Chlorine atoms are attached to the basic structure at any of 8 different places on the molecule, positions 1–4 and 6–9. There are 210 different types of PCDD/F congeners (herein, a congener means a related dioxin compound) comprising of 75 PCDDs and 135 PCDFs. The toxicity of PCDD/Fs depends on the number and position of the chlorine atoms; only congeners that have chlorines in the 2, 3, 7, and 8 positions have any observable toxicity. Out of the 210 PCDD/F compounds in total, only 17 congeners (7 PCDDs and 10 PCDFs) have chlorine atoms in the relevant positions to be considered toxic by the NATO Committee on the Challenges to Modern Society (NATO/CCMS) international toxic equivalent (I-TEQ) scheme.
Historical perspective
Dioxins did not exist in nature prior to industrialization, except in very trace amounts due to natural combustion and geological processes[2][3]. The first synthesis of dioxin dates back to 1872. Today they are found in all humans, with higher levels commonly found in persons living in more industrialized countries. The most toxic dioxin, 2,3,7,8 tetrachlorodibenzo-p-dioxin (TCDD), became well known as a contaminant of Agent Orange, an herbicide used in the Vietnam War[4]. Later dioxins were found in Times Beach, Missouri[5] and Love Canal, New York of USA[6] and Seveso, Italy[7]. Dioxins have already been in the news with poisoning of President Viktor Yushchenko of Ukraine in 2004.
Toxicity
Dioxins are carcinogenic in higher amounts and cause developmental and reproductive problems. They are absorbed primarily through the dietary intake of fat, as this is where they accumulate in animals and humans. In humans, the highly chlorinated dioxins are stored in fatty tissues and are neither readily metabolized nor excreted. This persistence is the consequence of their structure. In contrast, dioxins with few chlorines always contain hydrogen atoms on the adjacent pairs of carbons at which hydroxyl groups can readily be added in the biochemical reactions and tend to be excreted rather than stored for long time.
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is the most toxic of the congeners. Other dioxin congeners (or mixtures thereof) are given a toxicity rating from 0 to 1, where TCDD = 1. This toxicity rating is called the Toxic Equivalence Factor, or TEF. TEFs are consensus values and, because of the strong species dependence for toxicity, are listed separately for mammals, fish and birds. TEFs for mammalian species are generally applicable to human risk calculations. The TEFs have been developed from detailed assessment of literature data to facilitate both risk assessment and regulatory control.[8] Many other compounds may also have dioxin-like properties, particularly non-ortho PCBs, some of which can have TEFs as high as 0.1.
The total dioxin toxic equivalency (TEQ) value expresses the toxicity as if the mixture were pure TCDD. The TEQ approach and current TEFs have been adopted internationally as the most appropriate way to estimate the potential health risks of mixture of dioxins.
Dioxins and other persistent organic pollutants (POPs) are subject to the Stockholm Convention. The treaty obliges signatories to take measures to eliminate where possible, and minimize where not possible to eliminate, all sources of dioxin.
Sources of dioxin
The United States Environmental Protection Agency Dioxin Reassessment Report is possibly the most comprehensive review of dioxin, but other countries now have substantial research. Australia, New Zealand and the United Kingdom all have substantial research into body burdens and sources. Tolerable daily, monthly or annual intakes have been set by the World Health Organization and a number of governments. Dioxin enters the general population almost exclusively from ingestion of food, specifically through the consumption of fish, meat, and dairy products since dioxins are fat-soluble and readily climb the food chain.[9]
Occupational exposure is an issue for some in the chemical industry, or in the application of chemicals, notably herbicides. Inhalation has been a problem for people living near substantial point sources where emissions are not adequately controlled. In many developed nations there are now emissions regulations which have alleviated some concerns, although the lack of constant sampling of dioxin emissions causes concern about the understatement of emissions. In Belgium, through the introduction of a process called AMESA, constant sampling showed that periodic sampling understated emissions by a factor of 30 to 50 times. Few facilities have constant sampling.
Most controversial is the US EPA assessment's (draft) finding that any reference dose that were to be set would be far below current average intakes.
Children are passed substantial body burdens by their mothers, and breast feeding increases the child's body burden. Children's body burdens are often many times above the amount implied by tolerable intakes which are based on body weight. Breast fed children usually have substantially higher dioxin body burdens than non breast fed children until they are about 8 to 10 years old. The WHO still recommends breast feeding for its other benefits.
Dioxins are produced in small concentrations when organic material is burned in the presence of chlorine, whether the chlorine is present as chloride ions or as organochlorine compounds, so they are widely produced in many contexts. According to the most recent US EPA data the major sources of dioxin are:
- Coal fired utilities
- Metal smelting
- Diesel trucks
- Land application of sewage sludge
- Burning treated wood
- Trash burn barrels
These sources together account for nearly 80% of dioxin emissions.
Dioxins are also in smoke from typical cigarettes, those with chlorine-bleached paper and residues of many chlorine pesticides. Dioxin in cigarette smoke was noted as "understudied" by the US EPA in its "Re-Evaluating Dioxin" (1995). In that same document, the US EPA acknowledged that dioxin is "anthropogenic" (man-made, "not likely in nature"). Dioxin cannot come from the tobacco or any natural plant. Since then, the USA classified dioxin as a Known Human Carcinogen, and the USA signed the Stockholm Convention on Persistent Organic Pollutants(POPs) to globally phase out dioxin and 11 other of the worst industrial pollutants, though the treaty has not been ratified by the Senate. Nevertheless, chlorine tobacco pesticides and chlorine-bleached cigarette papers remain legal, with no warning required to consumers.
In incineration, dioxins can also reform in the atmosphere above the stack as the exhaust gases cool through a temperature window of 600 to 200°C. The most common method of reducing dioxins reforming or forming de novo is through rapid (30 millisecond) quenching of the exhaust gases through that 400°C window.[10] Incinerator emissions of dioxins have been reduced by over 90% as a result of new emissions control requirements. Incineration is now a very minor contributor to dioxin emissions.
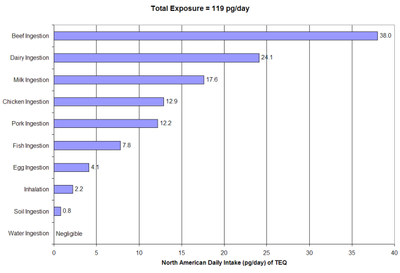
Dioxins are also generated in reactions that do not involve burning — such as bleaching fibers for paper or textiles, and in the manufacture of chlorinated phenols, particularly when reaction temperature is not well controlled. Affected compounds include the wood preservative pentachlorophenol, and also herbicides such as 2,4-dichlorophenoxyacetic acid (or 2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T). Higher levels of chlorination require higher reaction temperatures and greater dioxin production. See Agent Orange for more on contamination problems in the 1960s. Dioxins may also be formed during the photochemical breakdown of the common antimicrobial compound triclosan.[11]
Dioxins are present in minuscule amounts in a wide range of materials used by humans — including practically all substances manufactured using plastics, resins or bleaches.[citation needed] Such materials include tampons, and a wide variety of food packaging substances[citation needed]. The use of these materials means that all Western humans receive at least a very small daily dose of dioxin—however, it is disputed whether such exceptionally tiny exposures have any clinical relevance. It is even controversially discussed if dioxins might have a non-linear dose-response curve with beneficial health effects in a certain lower dose range, a phenomenon called hormesis.
Dietary sources of dioxin in the United States have been analyzed by the EPA and other scientists. Summaries of the primary dietary sources are in the following two graphics (note pg = picogram, or one trillionth of a gram, or 10−12, or 0.000000000001 g).[12]
Health effects in humans
Dioxins build up primarily in fatty tissues over time (bioaccumulate), so even small exposures may eventually reach dangerous levels. In 1994, EPA reported that dioxin is a probable carcinogen, but notes that non-cancer effects (reproduction and sexual development, immune system) may pose an even greater threat to human health. TCDD, the most toxic of the dibenzodioxins, has a half-life of approximately 7 years in humans, but at high concentrations, the elimination rate is enhanced by metabolism.[13] The health effects of dioxins are mediated by their action on a cellular receptor, the Aryl Hydrocarbon Receptor (AhR).[14]
Dioxins also accumulate in food chains in a fashion similar to other chlorinated compounds (bioaccumulate). This means that even small concentrations in contaminated water can be concentrated up a food chain to dangerous levels due to the long biological half life and low solubility of dioxins.
Exposure to high levels of dioxin in humans causes a severe form of persistent acne, known as chloracne [3] and hyperpigmentation. Other effects in humans may include:
- Central and Peripheral Nervous System pathology[citation needed]
- Thyroid disorders[citation needed]
- Damage to the Immune systems.[17]
- Endometriosis[citation needed]
- Diabetes[citation needed]
Health effects in other animals
While it has been difficult to prove that dioxins cause specific health effects in humans due to the lack of controlled dose experiments, studies in animals have shown that dioxin causes a wide variety of toxic effects. In particular, TCDD has been shown to be teratogenic, mutagenic, carcinogenic, immunotoxic, and hepatotoxic. Furthermore, alterations in multiple endocrine and growth factor systems have been reported. The most sensitive effects, observed in multiple species, appear to be developmental, including effects on the developing immune, nervous, and reproductive systems [18]. These effects are caused at body burdens close to those reported in humans.
Among the animals for which TCDD toxicity has been studied, there is strong evidence for the following effects:
- Birth defects (teratogenicity)
- In rodents, including rats [19], mice [20], hamsters and guinea pigs [21]; birds [22]; and fish [23].
- Cancer (including neoplasms in the mammalian lung, oral/nasal cavities, thyroid and adrenal glands, and liver, squamous cell carcinoma, and various animal hepatocarcinomas)
- Hepatotoxicity (liver toxicity)
- Endocrine disruption
- In rodents[citation needed] and fish [30]
- Immunosuppression
- In rodents[citation needed] and fish[citation needed].
- Learning [31]
Studies of dioxin's effects in Vietnam
US veterans' groups and Vietnamese groups, including the Vietnamese government, have convened scientific studies to explore their belief that dioxins were responsible for a host of disorders, including tens of thousands of birth defects in children, amongst Vietnam veterans as well as an estimated one million Vietnamese, through their exposure to Agent Orange during the Vietnam War, which was found to be highly contaminated with TCDD. Several exposure studies showed that some US Vietnam Veterans who were exposed to Agent Orange had serum TCDD levels up to 600 ppt in many years after they left Vietnam, compared to general population levels of approximately 1 to 2 ppt of TCDD. In Vietnam TCDD levels up to 1,000,000 ppt have been found in soil or sediments from Agent Orange contaminated areas 3 to 4 decades after spraying. In addition, elevated levels have been measured in food and wildlife in Vietnam[32] The most recent study, paid for by the National Academy of Sciences, was released in an April 2003 report. This report is currently (March 2007) being revised for release again later in 2007.
The Center for Disease Control found that dioxin levels in Vietnam veterans[33] were in no way atypical when compared against the rest of the population. The only exception existed for those who directly handled Agent Orange. These were members of Operation Ranch Hand. Long term studies of the members of Ranch Hand have thus far uncovered a possibility of elevated risks of diabetes.
Dioxin exposure incidents
- In 1949 in herbicide production plant for 2,4,5-T in Nitro, West Virginia 240 people were affected when a relief valve opened.[34]
- In 1963 a dioxin cloud escapes after an explosion in a Philips-Duphar plant (now Solvay Group) near Amsterdam. In the 1960s Philips-Duphar produced 2250 tonnes of 'Agent Orange' for the US Army.
- In 1976 large amounts of dioxin were released in an industrial accident at Seveso, although no immediate human fatalities or birth defects occurred.[35][36][37]
- In 1978, dioxin was one of the contaminants that forced the evacuation of the Love Canal neighborhood of Niagara Falls, New York. Dioxin also caused the 1983 evacuation of Times Beach, Missouri.
- In the 1960s, parts of the Spolana chemical plant in Neratovice, Czechoslovakia, were heavily contaminated by dioxins, when the herbicide 2,4,5-T (also a component of Agent Orange) was produced there. Workers in this factory were exposed to high concentrations of dioxins at that time. Dozens of them fell seriously ill. A possibly large amount of dioxins was flushed from the factory into the Labe river during the 2002 European flood. No direct consequences of this incident have thus far been recorded.
- In May 1999, there was a dioxin crisis in Belgium: quantities of dioxin had entered the food chain through contaminated animal feed. 7,000,000 chickens and 60,000 pigs had to be slaughtered. This scandal was followed by a landslide change in government in the elections one month later.
- On September 11, 2001 explosions released massive amounts of dust into the air. The air was measured for dioxin from September 23, 2001 to November 21, 2001 and reported to be "likely the highest ambient concentration that have ever been reported." [in history]. The EPA report dated October 2002 and released in December of 2002 titled "Exposure and Human Health Evaluation of Airborne Pollution from the World Trade Center Disaster" authored by the EPA Office of Research and Development in Washington states that dioxin levels recorded at a monitoring station on Park Row near City Hall Park in New York between October 12 and 29, 2001 averaged 5.6 parts per trillion/per cubic meter of air, or nearly six times the highest dioxin level ever recorded in the U.S. Dioxin levels in the rubble of the World Trade Centers was much higher with concentrations ranging from 10 to 170 parts per trillion. The report did no measuring of the toxicity of indoor air.
- In a 2001 case study,[38] physicians reported clinical changes in a 30 year old woman who had been exposed to a massive dosage (144,000 pg/g blood fat) of dioxin equal to 16,000 times the normal body level; the highest dose of dioxin ever recorded in a human. She suffered from chloracne, nausea, vomiting, epigastric pain, loss of appetite, leukocytosis, anemia, amenorrhoea and thrombocytopenia. However, other notable laboratory tests, such as immune function tests, were relatively normal. The same study also covered a second subject who had received a dosage equivalent to 2,900 times the normal level, who apparently suffered no notable negative effects other than chloracne. These patients were provided with olestra to accelerate dioxin elimination.[39]
- In 2004, a notable individual case of dioxin poisoning, Ukrainian politician Viktor Yushchenko was exposed to the second-largest measured dose of dioxins, according to the reports of the physicians responsible for diagnosing him. This is the first known case of a single high dose of TCDD dioxin poisoning, and was diagnosed only after a toxicologist recognised the symptoms of chloracne while viewing television news coverage of his condition.[40]
- In the early 2000s, residents of the city of New Plymouth, New Zealand, report many illnesses of people living around and working at the Dow Chemical plant. This plant ceased production of 2,4,5-T in 1987.
Incineration and dioxin emissions
It is claimed that modern waste incinerators—like Texas Industries' cement plant in Midlothian, Texas—are equipped with pollution control equipment which reduces dioxin emissions to insignificant levels. (However, the emissions from TXI's Midlothian plant have increased.[41][42]) Incineration of municipal solid waste, medical waste, sewage sludge, and hazardous waste together produce less than 3% of all dioxin emissions. When the original US EPA inventory of dioxin sources was done in 1987, incineration represented over 80% of known dioxin sources. As a result, US EPA implemented new emissions requirements. These regulations have been very successful in reducing dioxin stack emissions from incinerators.
However, there is debate over how "clean" this has made incineration, since the process of removing dioxin from stack emissions transfers dioxin residues to filter cake, slag and fly ash, toxic waste which still has to be disposed of safely.
References
- ^
Sandermann, W., Stockmann, H., Casten, R. (1957). "Über die Pyrolyse des Pentachlorphenols (Pyrolysis of pentachlorophenol)". Chemische Berichte. 90 (5): 690–692. doi:10.1002/cber.19570900506.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
"Compilation of EU Dioxin Exposure and Health Data" (.pdf). European Commission DG Environment. 1999.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^
"Questions and Answers About Dioxins".
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^
Schecter A. Birnbaum L., Ryan J. J., Constable J. D. (2006). "Dioxins: An overview". Environmental Research. 101 (3): 419–428. doi:10.1016/j.envres.2005.12.003.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
"Times Beach Record of Decision Signed". EPA. September 30, 1998.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^
"Love Canal Record of Decision Signed". EPA. October 26, 1987.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^
De Marchi, B.; et al. "Seveso, A Paradoxical Classical Disaster".
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help); Explicit use of et al. in:|last=(help) - ^
M. Van den Berg, L. S. Birnbaum, M. Denison, M. De Vito, W. Farland, M. Feeley, H. Fiedler, H. Hakansson, A. Hanberg, L. Haws, Martin Rose, S. Safe, D. Schrenk, C. Tohyama, A. Tritscher, J. Tuomisto, M. Tysklind, N. Walker, Richard E. Peterson (2006). "The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds". Toxicology Science. 93: 223–241. doi:10.1093/toxsci/kfl055. PIM 16829543.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, Silver A, Schmitz M. (2001). "Intake of dioxins and related compounds from food in the U.S. population". Journal of Toxicology and Environmental Health, Part A. 11: 1–18.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gordon McKay (2002). "Dioxin, formation and minimisation during municipal solid waste (MSW) incineration: review". Chemical Engineering Journal. 86: 343–368. doi:10.1016/S1385-8947(01)00228-5.
- ^
Latch D. E., Packer J. L., Stender B. L., VanOverbeke J., Arnold W. A., McNeill K. (2005). "Aqueous photochemistry of triclosan: formation of 2,4-dichlorophenol, 2,8-dichlorodibenzo-p-dioxin, and oligomerization products". Environmental toxicology and chemistry. 24 (3): 517–525. doi:10.1897/04-243R.1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, Silver A, Schmitz M. (2001). "Intake of dioxins and related compounds from food in the U.S. population". Journal of Toxicology and Environmental Health, Part A. 11: 1–18.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Geusau A, Schmaldienst S, Derfler K, Papke O, Abraham K. (2002). "Severe 2,3,7,8-tetrachlorodibenzo- p-dioxin (TCDD) intoxication: kinetics and trials to enhance elimination in two patients". Arch Toxicol. 76 (5–6): 316–325. doi:10.1007/s00204-002-0345-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) . - ^ Mimura J, Fujii-Kuriyama Y (2006). "Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions". Biochem Pharmacol. 72 (4): 393–404. doi:10.1016/j.bcp.2006.01.017.
- ^
Satu Alaluusua, Pier Calderara, Pier Mario Gerthoux, Pirjo-Liisa Lukinmaa, Outi Kovero, Larry Needham, Donald G. Patterson Jr., Jouko Tuomisto, and Paolo Mocarelli (2004). "Developmental Dental Aberrations After the Dioxin Accident in Seveso". Environmental Health Perspectives. 112 (13): 1313–1318. doi:10.1289/ehp.6920.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Peterson R. E., Theobald H. M., Kimmel G. L. (1993). "Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons". Critical Review in Toxicology. 23: 283–335.
{{cite journal}}: Text "issue 3" ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://ehp.niehs.nih.gov/docs/2002/110p1169-1173baccarelli/abstract.html
- ^ Birnbaum LS, Tuomisto J. (2000). "Non-carcinogenic effects of TCDD in animals". Food Addit Contam. 17 (4): 275–288.
- ^ NTP (2006). "NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies)". Natl Toxicol Program Tech Rep Ser. 521.
- ^
Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, Abbott
BD. (1999). "Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice". Toxicological Sciences. 47 (1): 86–92.
{{cite journal}}: line feed character in|author=at position 79 (help)CS1 maint: multiple names: authors list (link) . - ^
Kransler KM, McGarrigle BP, Olson JR. (2007). "Comparative developmental toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the hamster, rat and guinea pig". Toxicology. 229 (3): 214–225. doi:10.1016/j.tox.2006.10.019.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^
Bruggeman V, Swennen Q, De Ketelaere B, Onagbesan O, Tona K, Decuypere E. (2003). "Embryonic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chickens: effects of dose and embryonic stage on hatchability and growth". Comp Biochem Physiol C Toxicol Pharmacol. 136 (1): 17–28. doi:10.1016/S1532-0456(03)00168-6.
{{cite journal}}: CS1 maint: multiple names: authors list (link) . - ^
Carney SA, Prasch AL, Heideman W, Peterson RE. (2006). "Understanding dioxin developmental toxicity using the zebrafish model". Birth Defects Res A Clin Mol Teratol. 76 (1): 7–18. doi:10.1002/bdra.20216.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ NTP (2006). "NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies)". Natl Toxicol Program Tech Rep Ser. 521.
- ^ NTP (1997). "Selected lesions of dioxin in laboratory rodents". Toxicol Pathol. 25 (1): 72–79.
- ^
Grinwis GC, Vethaak AD, Wester PW, Vos JG. (2000). "Toxicology of environmental chemicals in the flounder (Platichthys flesus) with emphasis on the immune system: field, semi-field (mesocosm) and laboratory studies". Toxicol Lett. 112–113: 289–301. doi:10.1016/S0378-4274(99)00239-8.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ NTP (1997). "Selected lesions of dioxin in laboratory rodents". Toxicol Pathol. 25 (1): 72–79.
- ^
El-Sabeawy F, Enan E, Lasley B. (2001). "Biochemical and toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin in immature
male and female chickens". Comp Biochem Physiol C Toxicol Pharmacol. 129 (4): 317–327. doi:10.1016/S1532-0456(01)00199-5.
{{cite journal}}: line feed character in|title=at position 81 (help)CS1 maint: multiple names: authors list (link) - ^
Zodrow JM, Stegeman JJ, Tanguay RL. (2004). "Histological analysis of acute toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD) in zebrafish". Aquat Toxicol. 66 (1): 25–38. doi:10.1016/j.aquatox.2003.07.002.
{{cite journal}}: CS1 maint: multiple names: authors list (link) . - ^
Heiden TK, Carvan MJ 3rd, Hutz RJ. (2006). "Inhibition of follicular development, vitellogenesis, and serum
17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin". Toxicological Sciences. 90 (2): 490–499. doi:10.1093/toxsci/kfj085.
{{cite journal}}: line feed character in|title=at position 64 (help)CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ Birnbaum LS, Tuomisto J. (2000). "Non-carcinogenic effects of TCDD in animals". Food Addit Contam. 17 (4): 275–288.
- ^
Olie, K., Schecter, A.; et al. (1989). "Chlorinated dioxin and dibenzofuran levels in food and wildlife samples in the North and South of Vietnam". Chemosphere. 19: 493–496.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^
CDC. "Veteran's Health Activities" (.pdf).
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Zack, J. A., Suskind, R. R. (1980). "The mortality experience of workers exposed to tetrachlorodibenzodioxin in a trichlorophenol process accident". J. Occup. Med. 22: 11–14.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.roche.com/com_his_sev-e.pdf
- ^ http://www.hse.gov.uk/comah/sragtech/caseseveso76.htm
- ^ http://www.chm.bris.ac.uk/motm/245t/245th/seveso.htm
- ^ http://ehp.niehs.nih.gov/members/2001/109p865-869geusau/geusau-full.html
- ^ Lancet (1999) 354(9186):1266–7
- ^ http://www.nature.com/news/2004/041122//pf/041122-8_pf.html
- ^ TECQ monitoring station [1]
- ^ latest US EPA report [2]
- Law & Order: Criminal Intent. "Beast" (2005) - A woman dies from dioxin poisoning.
External links
- "Dioxin Homepage" at ejnet.org/dioxin.
- "EPA: Exposure and Human Health Reassessment of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds National Academy Sciences (NAS) Review Draft", a 2005 report by the National Center for Environmental Assessment
- "Dioxins and Dioxin-like Compounds in the Food Supply: Strategies to Decrease Exposure", a 2003 report by the National Academy of Sciences
- National Pollutant Inventory - Dioxin and Furan Fact Sheet
- "Rhodes Remediation" Website about remediation of dioxin contaminated Homebush Bay and land in Rhodes, a suburb of Sydney, NSW, Australia. Union Carbide was the polluter. An epidemiological study found no evidence that dioxin contamination from the Rhodes site has resulted in increased cancer rates in the potentially exposed population living around the former manufacturing plant.
- "Researcher Dispels Myth of Dioxins and Plastic Water Bottles" Johns Hopkins Researcher explains the facts about Dioxin
- "Health Risks from Dioxin and Related Compounds: Evaluation of the EPA Reassessment" Includes discussion of methods of evaluating risk of low concentrations, and Toxic Equivalency
- "Dioxins in Cigarette Smoke". Archives of Environmental Health, Pg. 44 (3) : 171-4 May/Jun89
- Pesticide residues that are legal contaminants of tobacco
- Health effects of dioxins
- "Assessment of the Health Risks of Dioxins", a 1998 report by the World Health Organisation.
- A summary of the above report by GreenFacts.
- Environment and Health 5:87 The risks of dioxin to human health (Review article) Re-evaluation of the health hazards posed by dioxins
- Ind. Health 41(3): 149–157 (2003) Impact of Agent Orange exposure among Korean Vietnam veterans. Scientific article that corroborates the increased risk of diabetes among Korean Vietnam veterans.
- Env. Health Persp. 109(8): 865–869 (2001) Severe 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Intoxication: Clinical and Laboratory Effects. Case study of a TCDD poisoning.
- Regulatory Toxicology and Pharmacology 38(3): 378–388 (2003) Dioxin and cancer: a critical review. Review article that questiones the carcinogenity of dioxins.
- Synopsis on dioxins and PCBs (1999) Review article with some interesting tables and figures.
- Environmental Health Perspectives 112(13): 1265–1268 (2004) Dioxin Revisited: Developments Since the 1997 IARC Classification of Dioxin as a Human Carcinogen. Review article that provides evidence for the carcinogenity of dioxins.
- Treatment of dioxin poisoning with olestra.
- New Zealand Ministry of Health page on dioxins
- IARC monograph: "Polychlorinated Dibenzo-para-dioxins"
