Glioma: Difference between revisions
LeadSongDog (talk | contribs) →Experimental therapies: cite gnome |
LeadSongDog (talk | contribs) →Relative effectiveness of treatments: cite gnome |
||
| Line 124: | Line 124: | ||
===Relative effectiveness of treatments=== |
===Relative effectiveness of treatments=== |
||
A 2007 meta-analysis compared surgical resection and biopsy as the initial surgical management option. Results show that there is insufficient evidence to make a reliable decision.<ref>{{ |
A 2007 meta-analysis compared surgical resection and biopsy as the initial surgical management option. Results show that there is insufficient evidence to make a reliable decision.<ref>{{cite journal |
||
|author=Hart MG, Grant R, Metcalfe SE |
|||
| ⚫ | |||
|title=Biopsy versus resection for high grade glioma |
|||
|journal=Cochrane Database of Systematic Reviews |
|||
|year=2000 |
|||
|issue=2 |
|||
|id=CD002034 |
|||
|doi=10.1002/14651858.CD002034}}</ref> |
|||
For high-grade gliomas, a 2003 meta-analysis compared radiotherapy with radiotherapy and chemotherapy. It showed a small but clear improvement from using chemotherapy with radiotherapy.<ref>{{cite journal |
|||
|author=Stewart L, Burdett S; Glioma Meta-analysis Trialists Group (GMT) |
|||
|title=Chemotherapy for high-grade glioma |
|||
|journal=Cochrane Database of Systematic Reviews |
|||
|year=2002 |
|||
|issue=3 |
|||
|id=CD003913 |
|||
| ⚫ | |doi=10.1002/14651858.CD003913}}</ref> For Glioblastoma Multiforme, a 2008 meta-analysis showed that Temozolomide is an effective treatment for "prolonging survival and delaying progression as part of primary therapy without impacting on QoL and with a low incidence of early adverse events."<ref>{{doi|10.1002/14651858.CD007415}}</ref> |
||
==Causes== |
==Causes== |
||
Revision as of 19:54, 10 March 2010
| Glioma | |
|---|---|
| Specialty | Oncology, neurology, neurosurgery |
| Frequency | 0.0343% |
A glioma is a type of tumor that starts in the brain or spine. It is called a glioma because it arises from glial cells. The most common site of gliomas is the brain.[1]
Classification
Gliomas are classified by cell type, by grade, and by location.
By type of cell
Gliomas are named according to the specific type of cell they most closely resemble. The main types of gliomas are:
- Ependymomas — ependymal cells
- Astrocytomas — astrocytes - Glioblastoma multiforme is the most common astrocytoma.
- Oligodendrogliomas — oligodendrocytes
- Mixed gliomas, such as oligoastrocytomas, contain cells from different types of glia.
By grade
Gliomas are further categorized according to their grade, which is determined by pathologic evaluation of the tumor.
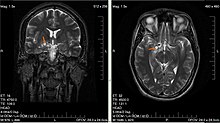
- Low-grade gliomas are well-differentiated (not anaplastic); these are benign and portend a better prognosis for the patient.
- High-grade gliomas are undifferentiated or anaplastic; these are malignant and carry a worse prognosis.
Of numerous grading systems in use, the most common is the World Health Organization (WHO) grading system for astrocytoma.
By location
Gliomas can be classified according to whether they are above or below a membrane in the brain called the tentorium. The tentorium separates the cerebrum, above, from the cerebellum, below.
- supratentorial: Above the tentorium, in the cerebrum, mostly in adults (70%). Senator Edward M. Kennedy’s brain tumor, for example was supratentorial, in the parietal area in the upper part of the left side of his brain, above the ear.[2]
- infratentorial: Below the tentorium, in the cerebellum, mostly in children (70%)
- Pontine: Located in the pons of the brainstem; The brain stem has three parts (Pons, midbrain and the medulla). The Pons controls critical functions such as breathing making surgery extremely dangerous.
Symptoms
Symptoms of gliomas depend on which part of the central nervous system is affected. A brain glioma can cause headaches, nausea and vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure. A glioma of the optic nerve can cause visual loss. Spinal cord gliomas can cause pain, weakness, or numbness in the extremities. Gliomas do not metastasize by the bloodstream, but they can spread via the cerebrospinal fluid and cause "drop metastases" to the spinal cord.
Pathology
High-grade gliomas are highly-vascular tumors and have a tendency to infiltrate. They have extensive areas of necrosis and hypoxia. Often tumor growth causes a breakdown of the blood-brain barrier in the vicinity of the tumor. As a rule, high-grade gliomas almost always grow back even after complete surgical excision.
On the other hand, low-grade gliomas grow slowly, often over many years, and can be followed without treatment unless they grow and cause symptoms.
Several acquired (not inherited) genetic mutations have been found in gliomas. TP53 is an early mutation. TP53 is the "guardian of the genome," which, during DNA and cell duplication, makes sure that the DNA is copied correctly and destroys the cell (apoptosis) if the DNA is mutated and can't be fixed. When TP53 itself is mutated, other mutations can survive. PTEN, another protein that also helps destroy cells with dangerous mutations, is itself lost or mutated. EGFR, a growth factor that normally stimulates cells to divide, is amplified and stimulates cells to divide too much. Together, these mutations lead to cells dividing uncontrollably, a hallmark of cancer. Recently, mutations in IDH1 and IDH2 were found to be part of the mechanism and associated with a more favorable prognosis.[3] The IDH1 and IDH2 genes are significant because they are involved in the citrate cycle in mitochondria. Mitochondria are involved in apoptosis. Furthermore, the altered glycolysis metabolism in some cancer cells leads to low oxygen (hypoxia). The normal response to hypoxia is to stimulate the growth of new blood vessels (angiogenesis). So these two genes may contribute to both the lack of apoptosis and vascularization of gliomas.
Prognosis
Gliomas cannot be cured. The prognosis for patients with high-grade gliomas is generally poor, and is especially so for older patients. Of 10,000 Americans diagnosed each year with malignant gliomas, about half are alive 1 year after diagnosis, and 25% after two years. Those with anaplastic astrocytoma survive about three years. Glioblastoma multiforme has a worse prognosis with less than 12 month survival after diagnosis.[4]
Treatment
Standard therapy
Treatment for brain gliomas depends on the location, the cell type and the grade of malignancy. Often, treatment is a combined approach, using surgery, radiation therapy, and chemotherapy. The radiation therapy is in the form of external beam radiation or the stereotactic approach using radiosurgery. Spinal cord tumors can be treated by surgery and radiation. Temozolomide is a chemotherapeutic drug that is able to cross the blood-brain barrier effectively and is being used in therapy.
Refractory disease
For recurrent high-grade glioblastoma, recent studies have taken advantage of angiogenic blockers such as bevacizumab in combination with conventional chemotherapy, with encouraging results.[5]
Experimental therapies
The use of oncolytic viruses or gene therapy using prodrug converting retroviruses and adenoviruses is being studied for the treatment of gliomas.[6][7]
The European Orphan Status Vaccine and Russian approved vaccine/drug Oncophage, or Vitespen is currently used at the Brain Tumor Research Center at the University of California, San Francisco, which has begun enrolling patients into a Phase 2 clinical trials in combination with the standard of care - radiation therapy plus Temodar (temozolomide) - for newly diagnosed glioma patients. The overall goals of the investigator-sponsored study are to evaluate median overall survival, progression-free survival and immunologic response to vaccine treatment.[8] The FDA has now set a provision allowing patients to receive such care using experimental drugs such as Oncophage to those in need with no other resource for care in the United States.
“To date, improvements in overall survival for newly diagnosed glioma patients have been negligible,” said Andrew T. Parsa, MD, PhD, associate professor in the department of neurological surgery at the University of California, San Francisco, and principal investigator of the trial. “The rationale for moving Oncophage into this patient population and combining it with radiation and Temodar was underscored by the encouraging results from the ongoing Phase 2 study in recurrent glioma, a more challenging patient population where the results showed overall survival increasing to approximately 10.5 months.”
The experimental cancer medicine "Ukrain" has been used for solid cancers. There are case reports of efficacy on gliomas.[9] Also under investigation is swainsonine.[10]
Most glioblastomas are infected with cytomegalovirus, and a clinical trial to immunize glioblastoma patients against cytomegalovirus resulted in slower growth of the tumors.[11]
5-aminolevulinic acid, a drug that makes certain cells, including gliomas, fluorescent, has been used to make surgical removal of gliomas more effective by making it easier to identify and remove them during surgery.[12]
Relative effectiveness of treatments
A 2007 meta-analysis compared surgical resection and biopsy as the initial surgical management option. Results show that there is insufficient evidence to make a reliable decision.[13] For high-grade gliomas, a 2003 meta-analysis compared radiotherapy with radiotherapy and chemotherapy. It showed a small but clear improvement from using chemotherapy with radiotherapy.[14] For Glioblastoma Multiforme, a 2008 meta-analysis showed that Temozolomide is an effective treatment for "prolonging survival and delaying progression as part of primary therapy without impacting on QoL and with a low incidence of early adverse events."[15]
Causes
The exact causes of gliomas are not known. Hereditary genetic disorders such as neurofibromatoses (type 1 and type 2) and tuberous sclerosis complex are known to predispose to their development[16]. Obesity during adolescence had a three to four times greater risk of developing glioma than having normal weight. Being tall also increased the risk; each 10 centimeter increase in height increased the risk nearly 20 percent. [17]
Notable cases
The following people are known to have been diagnosed with a glioma: Lee Atwater, Arleen Auger, Severiano Ballesteros, Fred Conlon, Ted Kennedy, Daniel W. Hardy, Dick Howser, Emlyn Hughes, George Gershwin, Gladys Marín, Tug McGraw, Robert Novak, Johnny Oates, Wolfram von Richthofen, Chuck Schuldiner, Kim Walker, Charles Whitman, Allen Shellenberger, Bobby Murcer.
In the movie Dark Victory (1939), the character Judith Traherne (played by Bette Davis) is diagnosed with glioma. (at 27:52 in the film)
In the movie No Way Out (1950), the character Johnnie Biddle dies of a glioma.
In the television series Buffy the Vampire Slayer, the character Joyce Summers (Buffy's Mom) played by Kristine Sutherland is diagnosed in Season 5 with low grade glioma and later dies from complications after surgery once it was removed.
References
- ^ Mamelak A.N., and Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin. Drug Drliv. (2007) 4(2):175-186.
- ^ Altman LK; O'Connor A (May 21, 2008). "Prognosis Usually Bleak for Condition, a Glioma". New York Times.
{{cite news}}: CS1 maint: multiple names: authors list (link) - ^ "IDH1 and IDH2 mutations in gliomas". New England Journal of Medicine. 360 (8): 765–73. 19 Feb 2009. doi:10.1056/NEJMoa0808710. PMC 2820383. PMID 19228619.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Malignant Gliomas Affect About 10,000 Americans Annually", By Rob Stein, Washington Post, May 20, 2008, http://www.washingtonpost.com/wp-dyn/content/article/2008/05/20/AR2008052001376.html
- ^ Wong ET, Brem S (2007). "Taming glioblastoma: targeting angiogenesis". J. Clin. Oncol. 25 (30): 4705–6. doi:10.1200/JCO.2007.13.1037. PMID 17947716.
- ^ Gromeier M, Wimmer E (2001). "Viruses for the treatment of malignant glioma". Curr. Opin. Mol. Ther. 3 (5): 503–8. PMID 11699896.
- ^ Rainov N, Ren H (2003). "Gene therapy for human malignant brain tumors". Cancer journal (Sudbury, Mass.). 9 (3): 180–8. PMID 12952303.
- ^ www.antigenics.com
- ^ Aschhoff B (2000). "Retrospective study of Ukrain treatment in 203 patients with advanced-stage tumors". Drugs Exp Clin Res. 26 (5–6): 249–52.
- ^ Sun JY, Yang H, Miao S, Li JP, Wang SW, Zhu MZ, Xie YH, Wang JB, Liu Z, Yang Q (2009). "Suppressive effects of swainsonine on C6 glioma cell in vitro and in vivo". Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 16 (11): 1070–4. doi:10.1016/j.phymed.2009.02.012. PMID 19427771.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Target acquired". The Economist. May 29, 2008.
- ^ Stummer W (2006). "Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial". Lancet Oncology. 7: 392–401. doi:10.1016/S1470-2045(06)70665-9.
- ^ Hart MG, Grant R, Metcalfe SE (2000). "Biopsy versus resection for high grade glioma". Cochrane Database of Systematic Reviews (2). doi:10.1002/14651858.CD002034. CD002034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stewart L, Burdett S; Glioma Meta-analysis Trialists Group (GMT) (2002). "Chemotherapy for high-grade glioma". Cochrane Database of Systematic Reviews (3). doi:10.1002/14651858.CD003913. CD003913.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ doi:10.1002/14651858.CD007415
- ^ PMID 19322539
- ^ Jennifer Thomas. Exercise in Adolescence May Cut Risk of Deadly Brain Tumor. HealthDay. October 6, 2009.
External links
- American Brain Tumor Association: Malignant Gliomas
- Brain and Spinal Tumors: Hope Through Research (National Institute of Neurological Disorders and Stroke)
- . GPnotebook https://www.gpnotebook.co.uk/simplepage.cfm?ID=-2147090429.
{{cite web}}: Missing or empty|title=(help) - Treatment Options for Glioblastoma and other Gliomas (.pdf format)
- German Brain Tumor Association
- WHO Classification of Glioma
- Glioma ImagesMedPix Database
- Experimental Anti-cancer Drug Kills Brain Tumor Stem Cells (Science Daily)
- Statin Plus Cancer Drug Deliver Combo Punch to Brain Cancer Cells (Medical News Today, Jan 2007)
- KGaA drug blasts aggressive brain tumours
- The latest news and medical research on gliomas via MedWorm
- Glioma brain tumor eliminated by Chinese medical research team using a novel new treatment
