Angiopoietin: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 67: | Line 67: | ||
[[:de:Angiopoetin]] |
[[:de:Angiopoetin]] |
||
[[:ru:Ангиопоэтин]] |
[[:ru:Ангиопоэтин]] |
||
==Introduction== |
|||
Angiopoietins are a family of vascular growth factors that play a role in embryonic and postnatal angiogenesis. It is responsible for assembling and disassembling the endothelial lining of blood vessels. <ref name="pmid20509945">{{cite journal| author=Alves BE, Montalvao SA, Aranha FJ, Siegl TF, Souza CA, Lorand-Metze I et al.| title=Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia. | journal=BMC Infect Dis | year= 2010 | volume= 10 | issue= | pages= 143 | pmid=20509945 | doi=10.1186/1471-2334-10-143 | pmc=PMC2890004 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20509945 }} </ref> Angiopoietin cytokines are involved with controlling microvascular permeability and allow the smooth muscle cells and smooth muscle-like pericytes to cover the vessels making vasodilation and vasoconstriction possible. <ref name="Gilbert2010">{{cite book|author=Scott F. Gilbert|title=Developmental Biology (Loose Leaf)|url=http://books.google.com/books?id=YtXLSAAACAAJ|date=10 April 2010|publisher=Sinauer Associates Incorporated|isbn=978-0-87893-558-1}}</ref> Angiopoietin signaling corresponds with the process of angiogenesis. Angiogenesis is the process by which new arteries and veins form from pre-existing blood cells. Overall, angiogenesis proceeds through sprouting, endothelial cell migration, proliferation, and vessel destabilization and stabilization. Angiopoietin-1 is critical for vessel maturation, adhesion, migration, and survival. Angiopoietin-2, on the other hand, promotes cell death and disrupts vascularization. Yet, when it is in conjunction with vascular endothelial growth factors, or VEGF, it can promote neo-vascularization. <ref name=Fagiani3>{{cite journal| author=Fagiani E, Christofori G| title=Angiopoietins in angiogenesis. | journal=Cancer Lett | year= 2013 | volume= 328 | issue= 1 | pages= 18-26 | pmid=22922303 | doi=10.1016/j.canlet.2012.08.018 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22922303 }} </ref> |
|||
==Specific Mechanisms of Angiopoietins == |
|||
The collective interactions between angiopoietins, tyrosine kinase receptors, vascular endothelial growth factors and their receptors form the two signaling pathways -- Tie-1 and Tie-2. They are mostly exclusive to endothelial cells and bind to tyrosine kinase receptor 2. |
|||
Angiopoietin proteins 1 through 4 are all ligands for the Tie-2 receptors. Tie-1 heterodimerizes with Tie-2 to enhance and modulate signal transduction of Tie-2 for vascular development and maturation. The tyrosine kinase receptors are typically expressed on vascular endothelial cells and specific macrophages for the immune response. <ref name=Fagiani3 /> Angiopoietin-1 is a growth factor produced by vascular support cells, specialized pericytes in the kidney, and hepatic stellate cells (ITO) cells in the liver. This growth factor is also a glycoprotein and functions as an agonist for the tyrosine receptor found in endothelial cells. <ref name="pmid21606590">{{cite journal| author=Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M et al.| title=Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. | journal=J Clin Invest | year= 2011 | volume= 121 | issue= 6 | pages= 2278-89 | pmid=21606590 | doi=10.1172/JCI46322 | pmc=PMC3104773 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21606590 }} </ref> Angiopoietin-1 and tyrosine kinase signaling are essential for regulating blood vessel development, and the stability of mature vessels. <ref name="pmid21606590" /> |
|||
Perivascular cells that coat mature vessels express angiopoietin-1, while angiopoietin-2 is expressed by endothelial cells. <ref name="pmid23500414">{{cite journal| author=Eklund L, Saharinen P| title=Angiopoietin signaling in the vasculature. | journal=Exp Cell Res | year= 2013 | volume= 319 | issue= 9 | pages= 1271-80 | pmid=23500414 | doi=10.1016/j.yexcr.2013.03.011 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23500414 }} </ref> This angiopoietin also maintains endothelial barrier integrity. <ref name="pmid20509945" /> |
|||
The expression of Angiopoietin-2 in the absence of VEGF leads to endothelial cell death and vascular regression. <ref>{{cite book | last = Harmey | first = Judith | title = VEGF and cancer | publisher = Landes Bioscience/Eurekah.com New York, N.Y. Kluwer Academic/Plenum Publishers | location = Georgetown, Tex | year = 2004 | isbn = 0-306-47988-5 }} </ref> Increased levels of Ang2 promote tumor angiogenesis, metastasis, and inflammation. Effective means to control Ang2 in inflammation and cancer should have clinical value.<ref name="pmid23500414" /> Angiopoeitin, more specifically Ang-1 and Ang-2, work hand in hand with vascular endothelial growth factor (VEGF) to mediate angiogenesis. Ang-2 works as an antagonist of Ang-1 and promotes vessel regression if VEGF isn’t present. Ang-2 works with VEGF to facilitate cell proliferation and migration of endothelial cells. <ref>{{cite journal| author=Lim HS, Blann AD, Chong AY, Freestone B, Lip GY| title=Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. | journal=Diabetes Care | year= 2004 | volume= 27 | issue= 12 | pages= 2918-24 | pmid=15562207 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15562207 }} </ref> |
|||
Angiopoietin-1 and angiopoietin-2 are modulators of endothelial permeability and barrier function. Endothelial cells secrete angiopoietin-2 for autocrine signaling while parenchymal cells of the extravascular tissue secrete angiopoietin-2 onto endothelial cells for paracrine signaling. It then binds to the extracellular matrix and is stored within the endothelial cells. <ref name=Yuan33>{{cite book|author1=Sarah Y. Yuan|author2=Robert R. Rigor|title=Regulation of Endothelial Barrier Function|url=http://books.google.com/books?id=E7FZPM4eRe8C|date=30 September 2010|publisher=Morgan & Claypool Publishers|isbn=978-1-61504-120-6}}</ref> |
|||
==Structure of Angiopoietins== |
|||
Angiopoietin proteins 1-4 are all ligands for the Tie-2 receptors. TIe-1 heterodimerizes with Tie-2 to enhance and modulate signal transduction of Tie-2. <ref name=Fagiani3 /> |
|||
Angiopoietin-1 encodes a 498 amino acid polypeptide with a molecular weight of 57 kDa whereas angiopoietin-2 encodes a 496 amino acid polypeptide. <ref name=Yuan33 /> |
|||
Structurally, angiopoietins have an N-terminal super clustering domain, a central coiled domain, a linker region, and a C terminal fibrinogen-related domain which is responsible for the binding between the ligand and receptor. Angiopoietin-1 and angiopoietin- 2 can form dimers, trimers, and tetramers. Angiopoietin-1 has the ability to form higher order multimers through its super clustering domain. However, not all of the structures can interact with the tyrosine kinase receptor. The tyrosine kinase receptor can only be activated at the tetramer level or higher. <ref name=Fagiani3 /> |
|||
==Clinical Relevance== |
|||
Deregulation of angiopoietin and the tyrosine kinase pathway is common in blood related diseases such as diabetes, malaria, sepsis, and pulmonary hypertension. This is demonstrated by an increased ratio of angiopoietin-2 and angiopoietin-1 in blood serum. Angiopoietin-2, is a produced in stored in Weibel-Palade bodies in endothelial cells, and acts as a Tek antagonist, causing the promotion of endothelial activation, destabilization as well as inflammation. Its role during angiogenesis depends on the presence of Vegf-a. <ref name="pmid21606590" /> |
|||
Angiopoietins are relevant in treating cancer as well. During tumor growth, pro-angiogenic molecules and anti-angiogenic molecules are off balance. Equilibrium is disrupted such that the pro-angiogenic molecules are increasing. Angiopoietins have been known to be recruited as well as VEGFs and PDGFs. Clinically, this is relevant for cancer treatments because the inhibition of angiogenesis can aid in suppressing tumor proliferation. <ref name="pmid19815705">{{cite journal| author=Falcón BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A et al.| title=Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. | journal=Am J Pathol | year= 2009 | volume= 175 | issue= 5 | pages= 2159-70 | pmid=19815705 | doi=10.2353/ajpath.2009.090391 | pmc=PMC2774078 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19815705 }} </ref> |
|||
Angiopoietins can also provide an indication on sepsis and a possible treatment. Angiopoietin levels are indicators for sepsis and can be clinically treated to prevent illness. Research on angiopoietin-2 has shown that it is involved in the onset of septic shock. With a fever onset and high levels of angiopoietin-2, the onset of septic shock is likely. It has also been shown that imbalances between angiopoietin-1 and angiopoietin-2 can act independently such that one might signal at high levels while the other remains at normal level signaling. <ref name="pmid20509945" /> |
|||
==References== |
|||
{{reflist}} |
|||
Revision as of 16:06, 4 November 2013
| angiopoietin 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | ANGPT1 | ||||||
| NCBI gene | 284 | ||||||
| HGNC | 484 | ||||||
| OMIM | 601667 | ||||||
| RefSeq | NM_001146 | ||||||
| UniProt | Q15389 | ||||||
| Other data | |||||||
| Locus | Chr. 8 q22.3-8q23 | ||||||
| |||||||
| angiopoietin 2 | |||||||
|---|---|---|---|---|---|---|---|
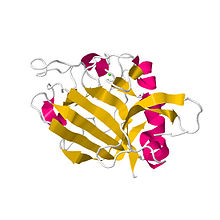 Crystal structure of the human angiopoietin-2 receptor binding domain.[1] | |||||||
| Identifiers | |||||||
| Symbol | ANGPT2 | ||||||
| NCBI gene | 285 | ||||||
| HGNC | 485 | ||||||
| OMIM | 601922 | ||||||
| RefSeq | NM_001147 | ||||||
| UniProt | O15123 | ||||||
| Other data | |||||||
| Locus | Chr. 8 p23 | ||||||
| |||||||
The angiopoietins are protein growth factors that promote angiogenesis, the formation of blood vessels from pre-existing blood vessels. There are now four identified angiopoietins: Ang1, Ang2, Ang3, Ang4. In addition, there are a number of proteins that are closely related to angiopoietins (ANGPTL2, ANGPTL3, ANGPTL4, ANGPTL5, ANGPTL6, ANGPTL7). Ang1 and Ang2 are required for the formation of mature blood vessels, as demonstrated by mouse knock out studies.[2]
Angiopoietin receptors
The TIE receptors are tyrosine kinases, so named because they mediate cell signals by inducing the phosphorylation of key tyrosines, thus initiating the binding and activation of downstream, intracellular enzymes; this process is called cell signalling, and it is the method by which cells are induced to activate or inhibit key regulatory functions. It is somewhat controversial which of the TIE receptors mediate functional signals downstream of Ang stimulation - but it is clear that at least TIE-2 is capable of physiologic activation as a result of binding the angiopoietins. The angiopoietins are the secreted ligands of the TIE family of protein receptor tyrosine kinases.
Clinical relevance
Angiopoietin 2 is elevated in patients with angiosarcoma.[3]
Vaccination against TIE-2-positive cells appears to reduce atheroma formation in experimental animals.[4]
There are changes in circulating levels and ratios of angiopoietins during pregnancy, but not during the menstrual cycle and in controlled ovarian hyperstimulation.[5]
References
- ^ PDB: 1Z3U; Barton WA, Tzvetkova D, Nikolov DB (2005). "Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition". Structure. 13 (5): 825–32. doi:10.1016/j.str.2005.03.009. PMID 15893672.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thurston G (2003). "Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis". Cell Tissue Res. 314 (1): 61–8. doi:10.1007/s00441-003-0749-6. PMID 12915980.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Amo Y, Masuzawa M, Hamada Y, Katsuoka K (2004). "Observations on angiopoietin 2 in patients with angiosarcoma". Br. J. Dermatol. 150 (5): 1028–9. doi:10.1111/j.1365-2133.2004.05932.x. PMID 15149523.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hauer AD, Habets KL, van Wanrooij EJ; et al. (2008). "Vaccination against TIE2 reduces atherosclerosis". Atherosclerosis. 204 (2): 365–71. doi:10.1016/j.atherosclerosis.2008.09.039. PMID 19022447.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hurliman AK, Speroff L, Stouffer RL, Patton PE, Lee A, Molskness TA (2010). "Changes in Circulating Levels and Ratios of Angiopoietins during Pregnancy, but not during the Menstrual Cycle and Controlled Ovarian Stimulation". Fertil. Steril. 93 (5): 1493–9. doi:10.1016/j.fertnstert.2009.04.036. PMC 2839053. PMID 19476937.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Angiopoietins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- "angiopoietin.de: endothelia activation from bench to bedside". Medical School Hannover. Retrieved 2009-01-22.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help)
Introduction
Angiopoietins are a family of vascular growth factors that play a role in embryonic and postnatal angiogenesis. It is responsible for assembling and disassembling the endothelial lining of blood vessels. [1] Angiopoietin cytokines are involved with controlling microvascular permeability and allow the smooth muscle cells and smooth muscle-like pericytes to cover the vessels making vasodilation and vasoconstriction possible. [2] Angiopoietin signaling corresponds with the process of angiogenesis. Angiogenesis is the process by which new arteries and veins form from pre-existing blood cells. Overall, angiogenesis proceeds through sprouting, endothelial cell migration, proliferation, and vessel destabilization and stabilization. Angiopoietin-1 is critical for vessel maturation, adhesion, migration, and survival. Angiopoietin-2, on the other hand, promotes cell death and disrupts vascularization. Yet, when it is in conjunction with vascular endothelial growth factors, or VEGF, it can promote neo-vascularization. [3]
Specific Mechanisms of Angiopoietins
The collective interactions between angiopoietins, tyrosine kinase receptors, vascular endothelial growth factors and their receptors form the two signaling pathways -- Tie-1 and Tie-2. They are mostly exclusive to endothelial cells and bind to tyrosine kinase receptor 2. Angiopoietin proteins 1 through 4 are all ligands for the Tie-2 receptors. Tie-1 heterodimerizes with Tie-2 to enhance and modulate signal transduction of Tie-2 for vascular development and maturation. The tyrosine kinase receptors are typically expressed on vascular endothelial cells and specific macrophages for the immune response. [3] Angiopoietin-1 is a growth factor produced by vascular support cells, specialized pericytes in the kidney, and hepatic stellate cells (ITO) cells in the liver. This growth factor is also a glycoprotein and functions as an agonist for the tyrosine receptor found in endothelial cells. [4] Angiopoietin-1 and tyrosine kinase signaling are essential for regulating blood vessel development, and the stability of mature vessels. [4] Perivascular cells that coat mature vessels express angiopoietin-1, while angiopoietin-2 is expressed by endothelial cells. [5] This angiopoietin also maintains endothelial barrier integrity. [1]
The expression of Angiopoietin-2 in the absence of VEGF leads to endothelial cell death and vascular regression. [6] Increased levels of Ang2 promote tumor angiogenesis, metastasis, and inflammation. Effective means to control Ang2 in inflammation and cancer should have clinical value.[5] Angiopoeitin, more specifically Ang-1 and Ang-2, work hand in hand with vascular endothelial growth factor (VEGF) to mediate angiogenesis. Ang-2 works as an antagonist of Ang-1 and promotes vessel regression if VEGF isn’t present. Ang-2 works with VEGF to facilitate cell proliferation and migration of endothelial cells. [7]
Angiopoietin-1 and angiopoietin-2 are modulators of endothelial permeability and barrier function. Endothelial cells secrete angiopoietin-2 for autocrine signaling while parenchymal cells of the extravascular tissue secrete angiopoietin-2 onto endothelial cells for paracrine signaling. It then binds to the extracellular matrix and is stored within the endothelial cells. [8]
Structure of Angiopoietins
Angiopoietin proteins 1-4 are all ligands for the Tie-2 receptors. TIe-1 heterodimerizes with Tie-2 to enhance and modulate signal transduction of Tie-2. [3] Angiopoietin-1 encodes a 498 amino acid polypeptide with a molecular weight of 57 kDa whereas angiopoietin-2 encodes a 496 amino acid polypeptide. [8] Structurally, angiopoietins have an N-terminal super clustering domain, a central coiled domain, a linker region, and a C terminal fibrinogen-related domain which is responsible for the binding between the ligand and receptor. Angiopoietin-1 and angiopoietin- 2 can form dimers, trimers, and tetramers. Angiopoietin-1 has the ability to form higher order multimers through its super clustering domain. However, not all of the structures can interact with the tyrosine kinase receptor. The tyrosine kinase receptor can only be activated at the tetramer level or higher. [3]
Clinical Relevance
Deregulation of angiopoietin and the tyrosine kinase pathway is common in blood related diseases such as diabetes, malaria, sepsis, and pulmonary hypertension. This is demonstrated by an increased ratio of angiopoietin-2 and angiopoietin-1 in blood serum. Angiopoietin-2, is a produced in stored in Weibel-Palade bodies in endothelial cells, and acts as a Tek antagonist, causing the promotion of endothelial activation, destabilization as well as inflammation. Its role during angiogenesis depends on the presence of Vegf-a. [4]
Angiopoietins are relevant in treating cancer as well. During tumor growth, pro-angiogenic molecules and anti-angiogenic molecules are off balance. Equilibrium is disrupted such that the pro-angiogenic molecules are increasing. Angiopoietins have been known to be recruited as well as VEGFs and PDGFs. Clinically, this is relevant for cancer treatments because the inhibition of angiogenesis can aid in suppressing tumor proliferation. [9] Angiopoietins can also provide an indication on sepsis and a possible treatment. Angiopoietin levels are indicators for sepsis and can be clinically treated to prevent illness. Research on angiopoietin-2 has shown that it is involved in the onset of septic shock. With a fever onset and high levels of angiopoietin-2, the onset of septic shock is likely. It has also been shown that imbalances between angiopoietin-1 and angiopoietin-2 can act independently such that one might signal at high levels while the other remains at normal level signaling. [1]
References
- ^ a b c Alves BE, Montalvao SA, Aranha FJ, Siegl TF, Souza CA, Lorand-Metze I; et al. (2010). "Imbalances in serum angiopoietin concentrations are early predictors of septic shock development in patients with post chemotherapy febrile neutropenia". BMC Infect Dis. 10: 143. doi:10.1186/1471-2334-10-143. PMC 2890004. PMID 20509945.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: PMC format (link) CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Scott F. Gilbert (10 April 2010). Developmental Biology (Loose Leaf). Sinauer Associates Incorporated. ISBN 978-0-87893-558-1.
- ^ a b c d Fagiani E, Christofori G (2013). "Angiopoietins in angiogenesis". Cancer Lett. 328 (1): 18–26. doi:10.1016/j.canlet.2012.08.018. PMID 22922303.
- ^ a b c Jeansson M, Gawlik A, Anderson G, Li C, Kerjaschki D, Henkelman M; et al. (2011). "Angiopoietin-1 is essential in mouse vasculature during development and in response to injury". J Clin Invest. 121 (6): 2278–89. doi:10.1172/JCI46322. PMC 3104773. PMID 21606590.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: PMC format (link) CS1 maint: multiple names: authors list (link) - ^ a b Eklund L, Saharinen P (2013). "Angiopoietin signaling in the vasculature". Exp Cell Res. 319 (9): 1271–80. doi:10.1016/j.yexcr.2013.03.011. PMID 23500414.
- ^ Harmey, Judith (2004). VEGF and cancer. Georgetown, Tex: Landes Bioscience/Eurekah.com New York, N.Y. Kluwer Academic/Plenum Publishers. ISBN 0-306-47988-5.
- ^ Lim HS, Blann AD, Chong AY, Freestone B, Lip GY (2004). "Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention". Diabetes Care. 27 (12): 2918–24. PMID 15562207.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Sarah Y. Yuan; Robert R. Rigor (30 September 2010). Regulation of Endothelial Barrier Function. Morgan & Claypool Publishers. ISBN 978-1-61504-120-6.
- ^ Falcón BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A; et al. (2009). "Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels". Am J Pathol. 175 (5): 2159–70. doi:10.2353/ajpath.2009.090391. PMC 2774078. PMID 19815705.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: PMC format (link) CS1 maint: multiple names: authors list (link)
