Trichophyton rubrum: Difference between revisions
Year cat |
major update based on Wikipedia Education Program project |
||
| Line 1: | Line 1: | ||
{{Taxobox |
{{Taxobox |
||
| image = Trichophyton_rubrum_microconidia.jpg |
|||
| status = |
|||
| ⚫ | |||
| image = Athletes.jpg |
|||
| image_width = 150px |
|||
| ⚫ | |||
| regnum = [[Fungus|Fungi]] |
| regnum = [[Fungus|Fungi]] |
||
| phylum = [[Ascomycota]] |
| phylum = [[Ascomycota]] |
||
| Line 13: | Line 11: | ||
| species = '''''T. rubrum''''' |
| species = '''''T. rubrum''''' |
||
| binomial = ''Trichophyton rubrum'' |
| binomial = ''Trichophyton rubrum'' |
||
| binomial_authority = |
| binomial_authority = (Castell.) Sabour. |
||
| synonyms = *''Trichophyton megninii'' <small>R. Blanch. (1895)</small> |
|||
*''Trichophyton fischeri'' <small>J. Kane (1977)</small> |
|||
*''Trichophyton raubitschekii'' <small>J. Kane, Salkin, Weitzman & Smitka (1982)</small> |
|||
*''Trichophyton kanei'' <small>Summerbell (1987)</small> |
|||
}} |
}} |
||
| ⚫ | |||
The growth rate of ''Trichophyton'' colonies in the lab can be slow to rather quick. Their texture is waxy, smooth and even to cottony. From the top, the color is white to bright yellowish beige or red violet. Reverse is pale, yellowish, brown, or reddish-brown. Although ''Trichophyton rubrum'' is the most common of the [[dermatophytes]] causing fingernail fungus infections, there are others. ''[[Trichophyton interdigitale]]'' is the second most common source of fungal nail infections from the dermatophyte group. (Used to be called ''Trichophyton mentagrophytes'' var ''interdigitale'' - new guidelines now state that a fungus can only be called ''T mentagrophytes'' if it is isolated from an animal - the human variant is ''T. interdigitale'') |
|||
'''''Trichophyton rubrum''''' is a [[dermatophyte|dermatophytic]] [[fungus]] in the phylum [[Ascomycota]], class [[Euascomycetes]]. It is an exclusively clonal,<ref name=Graser1999/> [[anthropophilic]] [[saprotroph]] that colonizes the upper layers of dead skin, and is the most common cause of [[athlete's foot]], fungal infection of nail, [[jock itch]], and [[ringworm]] worldwide.<ref name=Zaugg2009 /> ''Trichophyton rubrum'' was first described by [[Malmsten]] in 1845 and is currently recognized to be a complex of species that comprises multiple, geographically patterned morphotypes several of which have been formally described as distinct taxa, including ''T. violaceum'' (which is mainly found in Africa), ''T. raubitschekii'', ''T. gourvilii'', ''T. megninii'', ''T. soudanense'', ''T. violaceum''.<ref name=Williams1894/><ref name=Graser2008 /> |
|||
==Common skin diseases== |
|||
*[[Ringworm]] properly known as dermatophytosis |
|||
*[[Athlete's foot]] properly known as ''Tinea pedis'' |
|||
*[[Jock itch]] properly known as ''Tinea cruris'' |
|||
*Fungal [[folliculitis]] of the scalp properly known as ''[[Tinea capitis]]'' |
|||
*Fungal folliculitis of the beard properly known as ''[[Tinea barbae]]'' |
|||
*Fungal folliculitis of the legs properly known as ''[[Majocchi granuloma]]'' often occurs in females who shave their legs. |
|||
*[[Onychomycosis]] (nail infection) |
|||
==Growth and morphology== |
|||
==Identification== |
|||
[[Image:Trichophyton rubrum var. rodhaini PHIL 4248 lores.jpg|thumb|left|250px|Bottom view of a Sabouraud agar plate with a colony of ''Trichophyton rubrum'' var. ''rodhainii''.]]Typical isolates of ''T. rubrum'' are white and cottony on the surface. The colony underside is usually red, although some isolates appear more yellowish and others more brownish.<ref name=Kane1997 /> ''Trichophyton rubrum'' grows slowly in culture with sparse production of teardrop or peg-shaped [[microconidia]] laterally on fertile [[hyphae]]. Macroconidia, when present, are smooth-walled and narrowly club-shaped, although most isolates lack [[macroconidia]].<ref name=Kane1997 /> Growth is inhibited in the presence of certain sulfur-, nitrogen- and phosphorus-containing compounds. Isolates of ''T. rubrum'' are known to produce penicillin ''in vitro'' and ''in vivo''.<ref name=youssef1978 /> |
|||
[[Image:Trichophyton rubrum var. rodhaini PHIL 4248 lores.jpg|thumb|Bottom view of a Sabouraud agar plate with a colony of ''Trichophyton rubrum'' var. ''rodhaini''.]] |
|||
Positive, selective diagnosis of ''T. rubrum'' is difficult as many members of the genus react similarly with test reagents. The Mycology Unit at the Adelaide Women's and Children's Hospital uses a dermatophyte identification scheme comprising 6 different media to help identify and differentiate the various species and strains of ''Trichophyton''. The media in this scheme are Littman Oxgall agar, Lactritmel agar, Sabouraud's agar with 5% NaCl, 1% Peptone agar, Trichophyton agar No. 1, and hydrolysis of urea. |
|||
===Variants=== |
|||
Strains of ''T. rubrum'' form two distinct biogeographical subpopulations: one restricted to Africa, the population spread throughout the remainder of the world. Isolates of the African subpopulation report clinically as Tinea corporis and Tinea capitis.<ref name=Graser2008 /> In contrast, the globally-distributed subpopulation occurs predominantly in Tinea pedis and Tinea uniguium.<ref name=Graser2008 /> Members of the ''T. rubrum'' complex are endemic to different regions; isolates previously referred to ''T. megninii'' originate from Portugal, ''T. soudanense'' is found in Sub-Saharan Africa, ''T. violaceum'' was originally restricted to South Asia but has recently become more widespread in the global population. All species included in the ''T. rubrum'' complex are "–" mating type with the exception of ''T. megninii'' which represents the "+" mating type and is [[Auxotrophy|auxotrophic]] for [[histidine|<small>L</small>-histidine]].<ref name=Graser2008 /> The mating type identity of ''T. soudanense'' remains unknown.<ref name=Kane1997 /> ''Trichophyton raubitschekii'' is characterized by strongly granular colonies and is the only variant in the complex that produces urease.<ref name=Kane1997 /> |
|||
==Diagnostic tests== |
|||
{{multiple image |
|||
| align = right |
|||
| width = 250 |
|||
| image1 = Trichophyton_rubrum_colonies_800.jpg |
|||
| alt1 = Three tubes of growth medium, the left showing a cottony white colony, the middle with three greenish flat colones and the right with one flat red colony |
|||
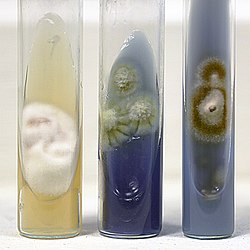
| caption1 = Colonies of ''T. rubrum ''isolated from toenail (left-right): Primary isolation from scrapings on Sabouraud's dextrose agar with cycloheximide, chloramphenicol and gentamicin (14 d); Greenish colonies on Littman Oxgall agar (14 d); Restricted, red colony without pH change on Bromocresol Purple Milk Solids Glucose agar (10 d). |
|||
| image2 = Mentag_rubrum_violaceum.jpg |
|||
| alt2 = Three tubes of opaque purple growth medium, the left showing a cottony white colony, the middle with a small red and the right with one with a small colony surrounded by a clear halo |
|||
| caption2 = Colonies of ''T. mentagrophytes'' (left), ''T. rubrum'' (center) and ''T. violaceum'' (right) showing differential responses on Bromocresol Purple Milk Solids Glucose agar (7 d). ''T. mentagrophytes'' shows unrestricted growth with alkaline (purple) colour change, ''T. rubrum'' shows restricted growth with no pH change, and ''T. violaceum'' produces weak growth accompanied by clearing of the milk solids and a purple colour change.}}Dermatophytes can usually be identified using microscopy; hair and nail scraping can be directly viewed under a microscope for identification. It can differentiated from other dermatophytes by the Bromocresol purple (BCP) milks as different Trichophyton species release different amounts of ammonia, ''T. rubrum'' will remain sky blue after 10 to 14 days indicating neutral pH.<ref name=Kane1997 /><ref name=Weitzman1995 /> However, contaminations can easily cause false positives when ''T. rubrum'' is grown on cycloheximide, as the distinctive red pigment in ''T. rubrum'' will not form in the absence of glucose.<ref name=Kane1997 /> Bacteria and saprotrophic fungi will out compete ''T. rubrum'' for glucose if they contaminate the sample, the red pigment can be restored using casamino acids erythritol agar (CEA), which reliably induces the formation the red pigment.<ref name=Kane1997 /> Cultures isolated using both cycloheximide-containing media and cycloheximide-free media are necessary for the verification of dermatophytic nail infections, especially when systemic treatment is being considered, given the propensity of these infections to involve non-dermatophytes.<ref name=Kane1997 /> A skin test is ineffective in diagnosing active infection and often yields false negative results.<ref name=Dahl1994 /> |
|||
==Pathology== |
|||
''Trichophyton rubrum'' is rarely isolated from animals.<ref name=Kane1997 /> In humans, men are more often infected than women.<ref name=DiSalvo1983 /> Infections can manifest as both chronic and acute forms.<ref name=Weitzman1995 /> Typically ''T. rubrum'' infections are restricted to the upper layers of the epidermis, however, deeper infections are possible.<ref name=Kwon-Chung1992 /> Approximately 80–93% of chronic dermatophyte infections are thought to be caused by ''T. rubrum'' including Tinea pedis, Tinea unguium, Tinea manuum, Tinea cruris, Tinea corporis, some cases of Tinea barbae have been also documented.<ref name=Graser2008 /> ''Trichophyton rubrum'' has also been known to cause [[folliculitis]] in which case it is characterized by fungal element in follicles and and foreign body giant cells in the dermis.<ref name=Weitzman1995 /> A ''T. rubrum'' infection may also form a [[granuloma]], extensive granuloma formations may occur in patients with immune deficiencies (e.g. [[Cushing syndrome]]). Immunodeficient neonates are susceptible to systemic ''T. rubrum'' infection.<ref name=Weitzman1995 /> |
|||
''Trichophyton rubrum'' infections do not elicit large inflammatory responses as this agent suppresses cellular immune responses involving [[lymphocytes]] particularly [[T cells]].<ref name=Weitzman1995 /> Mannan, a component of the fungal cell wall can also suppress immune responses although the mechanism of action remains unknown.<ref name=Dahl1994 /> ''Trichophyton rubrum'' infection has been associated with the induction of an [[id reaction]] in which an infection in one part of the body induces an immune response in the form of a sterile rash at a remote site.<ref name=Kane1997 /> The most common clinical forms of ''T. rubrum'' infection are described below. |
|||
===Foot=== |
|||
{{Main|Tinea pedis}} |
|||
''Trichophyton rubrum'' is one of the most common causes of chronic tinea pedis commonly known as athlete's foot.<ref name=DiSalvo1983 /> Chronic infections of tinea pedis result in [[moccasin foot]], in which the entire foot forms white scaly patches and infections usually affect both feet.<ref name=Weitzman1995 /> Individuals with Tinea pedis are likely to have infection at multiple sites.<ref name=DiSalvo1983 /> Infections can be spontaneously cured or controlled by topical antifungal treatment. Although ''T. rubrum'' Tinea pedis in children is extremely rare, it has been reported in children as young as 2 years of age.<ref name=Kwon-Chung1992 /> |
|||
===Hand=== |
|||
{{Main|Tinea manuum}} |
|||
Tinea manuum is commonly caused by ''T. rubrum'' and is characterized by unilateral infections of the palm of the hand.<ref name=Weitzman1995 /> |
|||
===Groin=== |
|||
{{Main|Tinea cruris}} |
|||
Along with ''E. floccosum'', ''T. rubrum'' is the most common cause of this disease also known as jock itch. Infections cause reddish brown lesions mainly on the upper thighs and trunk, that are border by raised edge.<ref name=Weitzman1995 /> |
|||
===Nail=== |
|||
{{Main|Onychomycosis}} |
|||
Once considered a rare causative agent,<ref name=DiSalvo1983 /> ''T. rubrum'' is now the most common cause of invasive fungal nail disease (called Onychomycosis or Tinea unguium).<ref name=Weitzman1995 /> Nail invasion by ''T. rubrum'' tends to be restricted to the underside of the nail plate and is characterized by the formation of white plaques on the [[Lunula (anatomy)|lunula]] that can spread to the entire nail. The nail often becomes thickens and brittle, turns the nail brown or black.<ref name=Kwon-Chung1992 /> Infections by ''T. rubrum'' are frequently chronic, remaining limited to the nails of only one or two digits for many years without progression.<ref name=DiSalvo1983 /> Spontaneous cure is rare.<ref name=DiSalvo1983 /> These infections are usually unresponsive to topical treatments and respond only poorly to systemic therapy.<ref name=Gohary2014 /> Although it is most frequently seen in adults, ''T. rubrum'' nail infections have been recorded in children.<ref name=DiSalvo1983 /> |
|||
==Epidemiology== |
|||
It is thought that ''Trichophyton rubrum'' evolved from a [[zoophilic]] ancestor, establishing itself ultimately as an exclusive agent of dermatophytosis on human hosts. Genetic analyses of ''T. rubrum'' have also revealed the presence of heat shock proteins, transporters, metabolic enzymes and a system of up regulation of key enzymes in the glyoxylate cycle.<ref name=Zaugg2009 /> It secretes more than 20 different [[protease]]s, including [[exopeptidase]]s and [[endopeptidase]]s that allow ''T. rubrum'' to digest human [[keratin]], [[collagen]] and [[elastin]], these proteases have an optimum pH of 8 and are calcium dependent.<ref name=Kwon-Chung1992 /> Although ''T. rubrum'' shares phylogenetic affiliations with other dermatophytes, it has a distinctive protein regulation system. |
|||
==Transmission== |
|||
This species has a propensity to infect glabrous (hairless) skin and is only exceptionally known from other sites.<ref name=Kwon-Chung1992 /> Transmission occurs via infected towels, linens, clothing (contributing factors are high humidity, heat, perspiration, diabetes mellitus, obesity, friction from clothes).<ref name=DiSalvo1983 /> Infection can be avoided by lifestyle and hygiene modifications such as avoiding walking barefoot on damp floors particularly in communal areas.<ref name=DiSalvo1983 /> |
|||
==Treatment== |
|||
Treatment depends on the locus and severity of infection. For Tinea pedis, many antifungal creams such as [[miconazole]] nitrate, [[clotrimazole]], [[tolnaftate]] (a synthetic thiocarbamate), [[terbinafine]] hydrochloride, butenafine hydrochloride and undecylenic acid are effective. For more severe or complicated infections, oral ketoconazole is an effective treatment for ''T. rubrum'' infections as this species exhibits greater susceptibility to this agent than other ''Trichophyton'' species.<ref name=docfungus /> Oral terbinafine, itraconazole or fluconazole have also all been shown to be effective treatments. Terbinafine and [[naftifine]] (topical creams) have been successfully treated Tinea cruris and Tinea corporis caused by ''T. rubrum''.<ref name=Gohary2014 /> Recently, general ''T. rubrum'' infection have been found to be susceptible to photodynamic treatment<ref name=Block1968 /> and laser irradiation.<ref name=Vural2007 /> |
|||
Tinea unguium presents a much greater therapeutic challenge as topical creams do not penetrate the nail bed. Systemic griseofulvin treatment have shown improvements in some patients with Tinea unguium; however, treatment failure is common in lengthy treatment courses (e.g., > 1 yr). Current treatment modalities have advocated intermittent "pulse therapy" with oral itraconazole<ref name=dedoncker1996/> of terbinafine.<ref name=gupta2014/> Fingernail infections can be treated in 6-8 weeks while toenail infections may take up to 12 weeks to achieve cure.<ref name=DiSalvo1983 /> Topical treatment by occlusive dressing combining 20% urea paste with 2% tolnaftate have also show promise in softening the nail plate to promote penetration of the antifungal agent to the nail bed.<ref name=DiSalvo1983 /> |
|||
==References== |
==References== |
||
{{Reflist|30em|refs= |
|||
{{reflist}} |
|||
<ref name=DiSalvo1983>{{cite book|last1=DiSalvo|first1=Edited by Arthur F.|title=Occupational mycoses|date=1983|publisher=Lea and Febiger|location=Philadelphia, Pa.|isbn=978-0812108859}}</ref> |
|||
<ref name=dedoncker1996>{{cite journal|last1=De Doncker|first1=P|last2=Decroix|first2=J|last3=Piérard|first3=GE|last4=Roelant|first4=D|last5=Woestenborghs|first5=R|last6=Jacqmin|first6=P|last7=Odds|first7=F|last8=Heremans|first8=A|last9=Dockx|first9=P|last10=Roseeuw|first10=D|title=Antifungal pulse therapy for onychomycosis. A pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1-week pulse therapy with itraconazole.|journal=Archives of dermatology|date=January 1996|volume=132|issue=1|pages=34-41|pmid=8546481}}</ref> |
|||
<ref name=Kwon-Chung1992> {{cite book|last1=Kwon-Chung|first1=K.J.|last2=Bennett|first2=John E.|title=Medical mycology|date=1992|publisher=Lea & Febiger|location=Philadelphia|isbn=9780812114638}}</ref> |
|||
<ref name=Kane1997> {{cite book|last1=Kane|first1=Julius|title=Laboratory handbook of dermatophytes : a clinical guide and laboratory handbook of dermatophytes and other filamentous fungi from skin, hair, and nails|date=1997|publisher=Star Pub.|location=Belmont, CA|isbn=978-0898631579}}</ref> |
|||
<ref name=Graser2008>{{cite journal|last1=Gräser|first1=Y|last2=Scott|first2=J|last3=Summerbell|first3=R|title=The new species concept in dermatophytes-a polyphasic approach|journal=Mycopathologia|date=2008|volume=166|issue=5–6|pages=239–56|pmid=18478366}}</ref> |
|||
<ref name=gupta2014>{{cite journal|last1=Gupta|first1=AK|last2=Daigle|first2=D|last3=Paquet|first3=M|title=Therapies for Onychomycosis: A Systematic Review and Network Meta-Analysis of Mycological Cure.|journal=Journal of the American Podiatric Medical Association|date=17 July 2014|pmid=25032982}}</ref> |
|||
<ref name=Graser1999>{{cite journal|last1=Gräser|first1=Y|last2=Kühnisch|first2=J|last3=Presber|first3=W|title=Molecular markers reveal exclusively clonal reproduction in Trichophyton rubrum.|journal=Journal of clinical microbiology|date=1999|volume=37|issue=11|pages=3713–7|pmid=10523582}}</ref> |
|||
<ref name=Block1968>{{cite journal|last1=Block|first1=PL|title=A wire-band splint for immobilizing loose posterior teeth.|journal=Journal of periodontology|date=1968|volume=39|issue=1|pages=17–8|pmid=5244503}}</ref> |
|||
<ref name=Vural2007>{{cite journal|last1=Vural|first1=Emre|last2=Winfield|first2=Harry L.|last3=Shingleton|first3=Alexander W.|last4=Horn|first4=Thomas D.|last5=Shafirstein|first5=Gal|title=The effects of laser irradiation on Trichophyton rubrum growth|journal=Lasers in Medical Science|date=2007|volume=23|issue=4|pages=349–353|doi=10.1007/s10103-007-0492-4|pmid=17902014}}</ref> |
|||
<ref name=Weitzman1995>{{cite journal|last1=Weitzman|first1=I|last2=Summerbell|first2=RC|title=The dermatophytes.|journal=Clinical microbiology reviews|date=1995|volume=8|issue=2|pages=240–59|pmid=7621400}}</ref> |
|||
<ref name=Gohary2014>{{cite journal|last1=El-Gohary|first1=M|last2=van Zuuren|first2=EJ|last3=Fedorowicz|first3=Z|last4=Burgess|first4=H|last5=Doney|first5=L|last6=Stuart|first6=B|last7=Moore|first7=M|last8=Little|first8=P|title=Topical antifungal treatments for tinea cruris and tinea corporis|journal=The Cochrane database of systematic reviews|date=2014|volume=8|pages=CD009992|pmid=25090020|doi=10.1002/14651858.CD009992.pub2}}</ref> |
|||
<ref name=Zaugg2009>{{cite journal|last1=Zaugg|first1=C|last2=Monod|first2=M|last3=Weber|first3=J|last4=Harshman|first4=K|last5=Pradervand|first5=S|last6=Thomas|first6=J|last7=Bueno|first7=M|last8=Giddey|first8=K|last9=Staib|first9=P|title=Gene expression profiling in the human pathogenic dermatophyte Trichophyton rubrum during growth on proteins|journal=Eukaryotic cell|date=2009|volume=8|issue=2|pages=241–50|pmid=19098130|pmc=2643602|display-authors=9|doi=10.1128/EC.00208-08}}</ref> |
|||
| ⚫ | |||
<ref name=docfungus>{{cite web|website=http://www.doctorfungus.org/thefungi/trichophyton.php}}</ref> |
|||
<ref name=Makimura 1999>{{cite journal|last1=Makimura|first1=Koichi|title=Phylogenetic Classification and Species Identification of Dermatophyte Strains Based on DNA Sequences of Nuclear Ribosomal Internal Transcribed Spacer 1 Regions|journal=Clinical Mycology|date=April|volume=4|issue=37|pages=920–924.|pmid=PMC88625}}</ref> |
|||
<ref name=Graser2008>{{cite journal|last1=Gräser|first1=Yvonne|last2=Scott|first2=James|last3=Summerbell|first3=Richard|title=The New Species Concept in Dermatophytes—a Polyphasic Approach|journal=Mycopathologia|date=2008|volume=166|issue=5–6|pages=239–256|doi=10.1007/s11046-008-9099-y|pmid=18478366}}</ref> |
|||
<ref name=Dahl1994>{{cite journal|last1=Dahl|first1=MV|last2=Grando|first2=SA|title=Chronic dermatophytosis: what is special about Trichophyton rubrum?|journal=Advances in dermatology|date=1994|volume=9|pages=97–109; discussion 110–1|pmid=8060745}}</ref> |
|||
<ref name=youssef1978>{{cite journal|last1=Youssef|first1=N|last2=Wyborn|first2=CH|last3=Holt|first3=G|title=Antibiotic production by dermatophyte fungi.|journal=Journal of general microbiology|date=March 1978|volume=105|issue=1|pages=105–111|pmid=632806}}</ref> |
|||
}} |
|||
==External links== |
|||
*[http://www.mycology.adelaide.edu.au/Fungal_Descriptions/Dermatophytes/Trichophyton/ Mycology Unit at the Adelaide Women's and Children's Hospital ] |
|||
*[http://www.doctorfungus.org/thefungi/Trichophyton.php Doctor Fungus] |
|||
{{Mycoses}} |
{{Mycoses}} |
||
Revision as of 19:32, 7 December 2014
| Trichophyton rubrum | |
|---|---|
| |
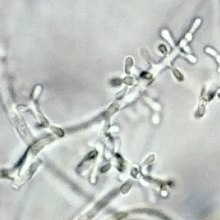
| Microconidia of T. rubrum | |
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Subphylum: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | T. rubrum
|
| Binomial name | |
| Trichophyton rubrum (Castell.) Sabour.
| |
| Synonyms | |
| |
Trichophyton rubrum is a dermatophytic fungus in the phylum Ascomycota, class Euascomycetes. It is an exclusively clonal,[1] anthropophilic saprotroph that colonizes the upper layers of dead skin, and is the most common cause of athlete's foot, fungal infection of nail, jock itch, and ringworm worldwide.[2] Trichophyton rubrum was first described by Malmsten in 1845 and is currently recognized to be a complex of species that comprises multiple, geographically patterned morphotypes several of which have been formally described as distinct taxa, including T. violaceum (which is mainly found in Africa), T. raubitschekii, T. gourvilii, T. megninii, T. soudanense, T. violaceum.[3][4]
Growth and morphology

Typical isolates of T. rubrum are white and cottony on the surface. The colony underside is usually red, although some isolates appear more yellowish and others more brownish.[5] Trichophyton rubrum grows slowly in culture with sparse production of teardrop or peg-shaped microconidia laterally on fertile hyphae. Macroconidia, when present, are smooth-walled and narrowly club-shaped, although most isolates lack macroconidia.[5] Growth is inhibited in the presence of certain sulfur-, nitrogen- and phosphorus-containing compounds. Isolates of T. rubrum are known to produce penicillin in vitro and in vivo.[6]
Variants
Strains of T. rubrum form two distinct biogeographical subpopulations: one restricted to Africa, the population spread throughout the remainder of the world. Isolates of the African subpopulation report clinically as Tinea corporis and Tinea capitis.[4] In contrast, the globally-distributed subpopulation occurs predominantly in Tinea pedis and Tinea uniguium.[4] Members of the T. rubrum complex are endemic to different regions; isolates previously referred to T. megninii originate from Portugal, T. soudanense is found in Sub-Saharan Africa, T. violaceum was originally restricted to South Asia but has recently become more widespread in the global population. All species included in the T. rubrum complex are "–" mating type with the exception of T. megninii which represents the "+" mating type and is auxotrophic for L-histidine.[4] The mating type identity of T. soudanense remains unknown.[5] Trichophyton raubitschekii is characterized by strongly granular colonies and is the only variant in the complex that produces urease.[5]
Diagnostic tests
Dermatophytes can usually be identified using microscopy; hair and nail scraping can be directly viewed under a microscope for identification. It can differentiated from other dermatophytes by the Bromocresol purple (BCP) milks as different Trichophyton species release different amounts of ammonia, T. rubrum will remain sky blue after 10 to 14 days indicating neutral pH.[5][7] However, contaminations can easily cause false positives when T. rubrum is grown on cycloheximide, as the distinctive red pigment in T. rubrum will not form in the absence of glucose.[5] Bacteria and saprotrophic fungi will out compete T. rubrum for glucose if they contaminate the sample, the red pigment can be restored using casamino acids erythritol agar (CEA), which reliably induces the formation the red pigment.[5] Cultures isolated using both cycloheximide-containing media and cycloheximide-free media are necessary for the verification of dermatophytic nail infections, especially when systemic treatment is being considered, given the propensity of these infections to involve non-dermatophytes.[5] A skin test is ineffective in diagnosing active infection and often yields false negative results.[8]
Pathology
Trichophyton rubrum is rarely isolated from animals.[5] In humans, men are more often infected than women.[9] Infections can manifest as both chronic and acute forms.[7] Typically T. rubrum infections are restricted to the upper layers of the epidermis, however, deeper infections are possible.[10] Approximately 80–93% of chronic dermatophyte infections are thought to be caused by T. rubrum including Tinea pedis, Tinea unguium, Tinea manuum, Tinea cruris, Tinea corporis, some cases of Tinea barbae have been also documented.[4] Trichophyton rubrum has also been known to cause folliculitis in which case it is characterized by fungal element in follicles and and foreign body giant cells in the dermis.[7] A T. rubrum infection may also form a granuloma, extensive granuloma formations may occur in patients with immune deficiencies (e.g. Cushing syndrome). Immunodeficient neonates are susceptible to systemic T. rubrum infection.[7]
Trichophyton rubrum infections do not elicit large inflammatory responses as this agent suppresses cellular immune responses involving lymphocytes particularly T cells.[7] Mannan, a component of the fungal cell wall can also suppress immune responses although the mechanism of action remains unknown.[8] Trichophyton rubrum infection has been associated with the induction of an id reaction in which an infection in one part of the body induces an immune response in the form of a sterile rash at a remote site.[5] The most common clinical forms of T. rubrum infection are described below.
Foot
Trichophyton rubrum is one of the most common causes of chronic tinea pedis commonly known as athlete's foot.[9] Chronic infections of tinea pedis result in moccasin foot, in which the entire foot forms white scaly patches and infections usually affect both feet.[7] Individuals with Tinea pedis are likely to have infection at multiple sites.[9] Infections can be spontaneously cured or controlled by topical antifungal treatment. Although T. rubrum Tinea pedis in children is extremely rare, it has been reported in children as young as 2 years of age.[10]
Hand
Tinea manuum is commonly caused by T. rubrum and is characterized by unilateral infections of the palm of the hand.[7]
Groin
Along with E. floccosum, T. rubrum is the most common cause of this disease also known as jock itch. Infections cause reddish brown lesions mainly on the upper thighs and trunk, that are border by raised edge.[7]
Nail
Once considered a rare causative agent,[9] T. rubrum is now the most common cause of invasive fungal nail disease (called Onychomycosis or Tinea unguium).[7] Nail invasion by T. rubrum tends to be restricted to the underside of the nail plate and is characterized by the formation of white plaques on the lunula that can spread to the entire nail. The nail often becomes thickens and brittle, turns the nail brown or black.[10] Infections by T. rubrum are frequently chronic, remaining limited to the nails of only one or two digits for many years without progression.[9] Spontaneous cure is rare.[9] These infections are usually unresponsive to topical treatments and respond only poorly to systemic therapy.[11] Although it is most frequently seen in adults, T. rubrum nail infections have been recorded in children.[9]
Epidemiology
It is thought that Trichophyton rubrum evolved from a zoophilic ancestor, establishing itself ultimately as an exclusive agent of dermatophytosis on human hosts. Genetic analyses of T. rubrum have also revealed the presence of heat shock proteins, transporters, metabolic enzymes and a system of up regulation of key enzymes in the glyoxylate cycle.[2] It secretes more than 20 different proteases, including exopeptidases and endopeptidases that allow T. rubrum to digest human keratin, collagen and elastin, these proteases have an optimum pH of 8 and are calcium dependent.[10] Although T. rubrum shares phylogenetic affiliations with other dermatophytes, it has a distinctive protein regulation system.
Transmission
This species has a propensity to infect glabrous (hairless) skin and is only exceptionally known from other sites.[10] Transmission occurs via infected towels, linens, clothing (contributing factors are high humidity, heat, perspiration, diabetes mellitus, obesity, friction from clothes).[9] Infection can be avoided by lifestyle and hygiene modifications such as avoiding walking barefoot on damp floors particularly in communal areas.[9]
Treatment
Treatment depends on the locus and severity of infection. For Tinea pedis, many antifungal creams such as miconazole nitrate, clotrimazole, tolnaftate (a synthetic thiocarbamate), terbinafine hydrochloride, butenafine hydrochloride and undecylenic acid are effective. For more severe or complicated infections, oral ketoconazole is an effective treatment for T. rubrum infections as this species exhibits greater susceptibility to this agent than other Trichophyton species.[12] Oral terbinafine, itraconazole or fluconazole have also all been shown to be effective treatments. Terbinafine and naftifine (topical creams) have been successfully treated Tinea cruris and Tinea corporis caused by T. rubrum.[11] Recently, general T. rubrum infection have been found to be susceptible to photodynamic treatment[13] and laser irradiation.[14]
Tinea unguium presents a much greater therapeutic challenge as topical creams do not penetrate the nail bed. Systemic griseofulvin treatment have shown improvements in some patients with Tinea unguium; however, treatment failure is common in lengthy treatment courses (e.g., > 1 yr). Current treatment modalities have advocated intermittent "pulse therapy" with oral itraconazole[15] of terbinafine.[16] Fingernail infections can be treated in 6-8 weeks while toenail infections may take up to 12 weeks to achieve cure.[9] Topical treatment by occlusive dressing combining 20% urea paste with 2% tolnaftate have also show promise in softening the nail plate to promote penetration of the antifungal agent to the nail bed.[9]
References
- ^ Gräser, Y; Kühnisch, J; Presber, W (1999). "Molecular markers reveal exclusively clonal reproduction in Trichophyton rubrum". Journal of clinical microbiology. 37 (11): 3713–7. PMID 10523582.
- ^ a b Zaugg, C; Monod, M; Weber, J; Harshman, K; Pradervand, S; Thomas, J; Bueno, M; Giddey, K; Staib, P (2009). "Gene expression profiling in the human pathogenic dermatophyte Trichophyton rubrum during growth on proteins". Eukaryotic cell. 8 (2): 241–50. doi:10.1128/EC.00208-08. PMC 2643602. PMID 19098130.
{{cite journal}}: Invalid|display-authors=9(help) - ^ William Williams, The Principles and Practice of Veterinary Surgery, p.734, W.R. Jenkins, 1894, from the collection of the University of California.
- ^ a b c d e Gräser, Y; Scott, J; Summerbell, R (2008). "The new species concept in dermatophytes-a polyphasic approach". Mycopathologia. 166 (5–6): 239–56. PMID 18478366. Cite error: The named reference "Graser2008" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j Kane, Julius (1997). Laboratory handbook of dermatophytes : a clinical guide and laboratory handbook of dermatophytes and other filamentous fungi from skin, hair, and nails. Belmont, CA: Star Pub. ISBN 978-0898631579.
- ^ Youssef, N; Wyborn, CH; Holt, G (March 1978). "Antibiotic production by dermatophyte fungi". Journal of general microbiology. 105 (1): 105–111. PMID 632806.
- ^ a b c d e f g h i Weitzman, I; Summerbell, RC (1995). "The dermatophytes". Clinical microbiology reviews. 8 (2): 240–59. PMID 7621400.
- ^ a b Dahl, MV; Grando, SA (1994). "Chronic dermatophytosis: what is special about Trichophyton rubrum?". Advances in dermatology. 9: 97–109, discussion 110–1. PMID 8060745.
- ^ a b c d e f g h i j k DiSalvo, Edited by Arthur F. (1983). Occupational mycoses. Philadelphia, Pa.: Lea and Febiger. ISBN 978-0812108859.
{{cite book}}:|first1=has generic name (help) - ^ a b c d e Kwon-Chung, K.J.; Bennett, John E. (1992). Medical mycology. Philadelphia: Lea & Febiger. ISBN 9780812114638.
- ^ a b El-Gohary, M; van Zuuren, EJ; Fedorowicz, Z; Burgess, H; Doney, L; Stuart, B; Moore, M; Little, P (2014). "Topical antifungal treatments for tinea cruris and tinea corporis". The Cochrane database of systematic reviews. 8: CD009992. doi:10.1002/14651858.CD009992.pub2. PMID 25090020.
- ^ http://www.doctorfungus.org/thefungi/trichophyton.php.
{{cite web}}: External link in|website=|title=(help); Missing or empty|url=(help) - ^ Block, PL (1968). "A wire-band splint for immobilizing loose posterior teeth". Journal of periodontology. 39 (1): 17–8. PMID 5244503.
- ^ Vural, Emre; Winfield, Harry L.; Shingleton, Alexander W.; Horn, Thomas D.; Shafirstein, Gal (2007). "The effects of laser irradiation on Trichophyton rubrum growth". Lasers in Medical Science. 23 (4): 349–353. doi:10.1007/s10103-007-0492-4. PMID 17902014.
- ^ De Doncker, P; Decroix, J; Piérard, GE; Roelant, D; Woestenborghs, R; Jacqmin, P; Odds, F; Heremans, A; Dockx, P; Roseeuw, D (January 1996). "Antifungal pulse therapy for onychomycosis. A pharmacokinetic and pharmacodynamic investigation of monthly cycles of 1-week pulse therapy with itraconazole". Archives of dermatology. 132 (1): 34–41. PMID 8546481.
- ^ Gupta, AK; Daigle, D; Paquet, M (17 July 2014). "Therapies for Onychomycosis: A Systematic Review and Network Meta-Analysis of Mycological Cure". Journal of the American Podiatric Medical Association. PMID 25032982.