Marine mammal: Difference between revisions
m →Classification of extant species: added binomial name of sealion to caption |
→Distribution and habitat: added Ecology section |
||
| Line 107: | Line 107: | ||
The first marine mammals appeared in the [[Jurassic]]. The teeth of ''[[Castorocauda]]'', a presumably beaver-like mammal, are different in many ways from all other [[docodont]]s, presumably due to a difference in diet. Most docodonts had teeth specialized for an [[omnivore|omnivorous]] diet. The teeth of ''Castorocauda'' suggest that the animal was a [[piscivore]], feeding on [[fish]] and small [[invertebrate]]s. The first two [[Molar (tooth)|molar]]s had cusps in a straight row, eliminating the grinding function suggesting that they were strictly for gripping and not for chewing. This feature of three cusps in a row is similar to the [[plesiomorphy|ancestral condition]] in mammal relatives (as seen in [[triconodont]]s), but is almost certainly a derived character in ''Castorocauda''. These first molars were also recurved in a manner designed to hold slippery prey once grasped. These teeth are very similar to the teeth seen in [[mesonychid]]s, an extinct group of semi-aquatic [[carnivore|carnivorous]] [[ungulate]]s, and resemble, to a lesser degree, the teeth of seals.<ref>{{cite journal|first1=Qiang|last1=Ji|first2=Zhe-Xi|last2=Luo|first3=Chong-Xi|last3=Yuan|first4=Alan R.|last4=Tabrum|year=2006|title=A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals|journal=Science|volume=311|issue=5764|pages=1123–1127|doi=10.1126/science.1123026|PMID=16497926}}</ref> |
The first marine mammals appeared in the [[Jurassic]]. The teeth of ''[[Castorocauda]]'', a presumably beaver-like mammal, are different in many ways from all other [[docodont]]s, presumably due to a difference in diet. Most docodonts had teeth specialized for an [[omnivore|omnivorous]] diet. The teeth of ''Castorocauda'' suggest that the animal was a [[piscivore]], feeding on [[fish]] and small [[invertebrate]]s. The first two [[Molar (tooth)|molar]]s had cusps in a straight row, eliminating the grinding function suggesting that they were strictly for gripping and not for chewing. This feature of three cusps in a row is similar to the [[plesiomorphy|ancestral condition]] in mammal relatives (as seen in [[triconodont]]s), but is almost certainly a derived character in ''Castorocauda''. These first molars were also recurved in a manner designed to hold slippery prey once grasped. These teeth are very similar to the teeth seen in [[mesonychid]]s, an extinct group of semi-aquatic [[carnivore|carnivorous]] [[ungulate]]s, and resemble, to a lesser degree, the teeth of seals.<ref>{{cite journal|first1=Qiang|last1=Ji|first2=Zhe-Xi|last2=Luo|first3=Chong-Xi|last3=Yuan|first4=Alan R.|last4=Tabrum|year=2006|title=A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals|journal=Science|volume=311|issue=5764|pages=1123–1127|doi=10.1126/science.1123026|PMID=16497926}}</ref> |
||
==Distribution and habitat== |
==Ecology== |
||
===Distribution and habitat=== |
|||
[[Image:PikiWiki Israel 15217 Dolphin.JPG|thumb|Bottlenose dolphin at [[Dolphin Reef]], [[Eilat]], [[Israel]]]] |
[[Image:PikiWiki Israel 15217 Dolphin.JPG|thumb|Bottlenose dolphin at [[Dolphin Reef]], [[Eilat]], [[Israel]]]] |
||
Marine mammals are widely distributed throughout the globe, but their distribution is patchy and coincides with the productivity of the oceans.<ref name="berta">{{cite book|last1=Berta|first1=A|last2=Sumich|first2=J. L.|year= 1999|title=Marine Mammals: Evolutionary Biology|location=San Diego|publisher=Academic Press|ISBN=978-0-12-093225-2|OCLC=42467530}}</ref> Species richness peaks at around 40° latitude, both north and south. This corresponds to the highest levels of [[primary production]] around North and South [[Americas|America]], [[Africa]], [[Asia]] and [[Australia]]. Total species range is highly variable for marine mammal species. On average most marine mammals have ranges which are equivalent or smaller than one-fifth of the [[Indian Ocean]].<ref name="ship"/> The variation observed in range size is a result of the different ecological requirements of each species and their ability to cope with a broad range of environmental conditions. There is a high degree of overlap between marine mammal species richness and areas of [[human impact on the environment]] which is of concern.<ref name=kasc/> |
Marine mammals are widely distributed throughout the globe, but their distribution is patchy and coincides with the productivity of the oceans.<ref name="berta">{{cite book|last1=Berta|first1=A|last2=Sumich|first2=J. L.|year= 1999|title=Marine Mammals: Evolutionary Biology|location=San Diego|publisher=Academic Press|ISBN=978-0-12-093225-2|OCLC=42467530}}</ref> Species richness peaks at around 40° latitude, both north and south. This corresponds to the highest levels of [[primary production]] around North and South [[Americas|America]], [[Africa]], [[Asia]] and [[Australia]]. Total species range is highly variable for marine mammal species. On average most marine mammals have ranges which are equivalent or smaller than one-fifth of the [[Indian Ocean]].<ref name="ship"/> The variation observed in range size is a result of the different ecological requirements of each species and their ability to cope with a broad range of environmental conditions. There is a high degree of overlap between marine mammal species richness and areas of [[human impact on the environment]] which is of concern.<ref name=kasc/> |
||
Most marine mammals, such as seals and sea otters, inhabit the coast. Seals, however, also use a number of terrestrial habitats, both continental and island. In temperate and tropical areas, they [[hauling-out|haul-out]] on to sandy and [[pebble]] beaches, [[rocky shore]]s, [[shoal]]s, [[mud flat]]s, [[tide pool]]s and in [[sea cave]]s. Some species also rest on man-made structures, like [[pier]]s, [[Jetty|jettie]]s, [[buoy]]s and [[oil platform]]s. Seals may move further inland and rest in sand dunes or vegetation, and may even climb cliffs.<ref name=thepinnipeds>{{cite book|author=Riedman, M.|year=1990|title=The Pinnipeds: Seals, Sea Lions, and Walruses|publisher=University of California Press|location=Los Angeles|isbn=978-0-520-06497-3|oclc=19511610}}</ref>{{rp|96}} Most cetaceans live in the open ocean, and species like the [[sperm whale]] may dive to depths of {{convert|-1000|to|-2500|ft|m}} in search of food.<ref>{{cite book|ref=Whitehead|title=Sperm Whales: Social Evolution in the Ocean|author=Whitehead, H.|year=2003|page=79|isbn=978-0-226-89518-5|oclc=51242162|publisher=University of Chicago Press|location=Chicago}}</ref> Sirenians live in shallow coastal waters, usually living {{convert|30|ft|m}} below sea level. However, they have been known to dive to {{convert|-120|ft|m}} to forage deep-water [[seagrass]]es.<ref>{{cite book|last1=Marsh|first1=H.|last2=Eros|first2= Carole|last3=Hugues|first3=Joanna|last4=Penrose|first4=Helen|year=2002|url=https://portals.iucn.org/library/sites/library/files/documents/2002-001.pdf|title=Dugong: status reports and action plans for countries and territories| publisher=International Union for Conservation of Nature|ISBN=978-92-807-2130-0|OCLC=51040880}}</ref> Freshwater variety, generally wade in the shallow parts of lakes, with a few exceptions, and are bottom-feeders.<ref name="Reidenberg" /> |
Most marine mammals, such as seals and sea otters, inhabit the coast. Seals, however, also use a number of terrestrial habitats, both continental and island. In temperate and tropical areas, they [[hauling-out|haul-out]] on to sandy and [[pebble]] beaches, [[rocky shore]]s, [[shoal]]s, [[mud flat]]s, [[tide pool]]s and in [[sea cave]]s. Some species also rest on man-made structures, like [[pier]]s, [[Jetty|jettie]]s, [[buoy]]s and [[oil platform]]s. Seals may move further inland and rest in sand dunes or vegetation, and may even climb cliffs.<ref name=thepinnipeds>{{cite book|author=Riedman, M.|year=1990|title=The Pinnipeds: Seals, Sea Lions, and Walruses|publisher=University of California Press|location=Los Angeles|isbn=978-0-520-06497-3|oclc=19511610}}</ref>{{rp|96}} Most cetaceans live in the open ocean, and species like the [[sperm whale]] may dive to depths of {{convert|-1000|to|-2500|ft|m}} in search of food.<ref>{{cite book|ref=Whitehead|title=Sperm Whales: Social Evolution in the Ocean|author=Whitehead, H.|year=2003|page=79|isbn=978-0-226-89518-5|oclc=51242162|publisher=University of Chicago Press|location=Chicago}}</ref> Sirenians live in shallow coastal waters, usually living {{convert|30|ft|m}} below sea level. However, they have been known to dive to {{convert|-120|ft|m}} to forage deep-water [[seagrass]]es.<ref>{{cite book|last1=Marsh|first1=H.|last2=Eros|first2= Carole|last3=Hugues|first3=Joanna|last4=Penrose|first4=Helen|year=2002|url=https://portals.iucn.org/library/sites/library/files/documents/2002-001.pdf|title=Dugong: status reports and action plans for countries and territories| publisher=International Union for Conservation of Nature|ISBN=978-92-807-2130-0|OCLC=51040880}}</ref> Freshwater variety, generally wade in the shallow parts of lakes, with a few exceptions, and are bottom-feeders.<ref name="Reidenberg" /> |
||
===Whale pump=== |
|||
{{Main|Whale pump|Whale fall}} |
|||
[[File:Oceanic whale pump - journal.pone.0013255.g001.tiff|thumb|"Whale pump" – the role played by whales in recycling ocean nutrients<ref name=pump/>]] |
|||
A 2010 study considered whales to be a positive influence to the productivity of ocean fisheries, in what has been termed a "[[whale pump]]". Whales carry nutrients such as nitrogen from the depths back to the surface. This functions as an upward biological pump, reversing an earlier presumption that whales accelerate the loss of nutrients to the bottom. This nitrogen input in the Gulf of Maine is "more than the input of all rivers combined" emptying into the gulf, some {{convert|23,000|MT|ST}} each year.<ref name=pump>{{cite journal|last1=Roman|first1=J.|last2=McCarthy|first2=J. J.|year=2010|editor-last=Roopnarine|editor-first=Peter|title=The Whale Pump: Marine Mammals Enhance Primary Productivity in a Coastal Basin|journal=PLoS ONE|volume=5|issue=10|doi=10.1371/journal.pone.0013255}}</ref> |
|||
[[Whale faeces|Whales defecate]] at the ocean's surface; their excrement is important for fisheries because it is rich in iron and nitrogen. The whale faeces are liquid and instead of sinking, they stay at the surface where [[phytoplankton]] feed off it.<ref name=pump/><ref>{{cite journal |title=Whales as marine ecosystem engineers |author1=Roman, Joe|author2=Estes, James A.|author3=Morissette, Lyne|author4=Smith, Craig|author5=Costa, Daniel|author6=McCarthy, James|author7=Nation, J.B.|author8=Nicol, Stephen|author9=Pershing, Andrew|author10=Smetacek, Victor |journal=Frontiers in Ecology and the Environment |volume=12|issue=7|pages=377–385|doi=10.1890/130220 |date=2014}}</ref> |
|||
Upon death, whale carcasses fall to the deep ocean and provide a substantial habitat for marine life. Evidence of [[whale fall]]s in present-day and fossil records shows that deep sea whale falls support a rich assemblage of creatures, with a global diversity of 407 species, comparable to other [[neritic]] biodiversity hotspots, such as [[cold seep]]s and [[hydrothermal vent]]s.<ref>{{cite journal|last1=Smith|first1=Craig R.|last2=Baco|first2=Amy R.|year=2003|title=Ecology of Whale Falls at the Deep-Sea Floor|journal=Oceanography and Marine Biology: An Annual Review|volume=41|pages=311–354 |url=http://www.soest.hawaii.edu/oceanography/faculty/csmith/Files/Smith%20and%20Baco%202003.pdf}}</ref> Deterioration of whale carcasses happens though a series of three stages. Initially, moving organisms such as [[shark]]s and [[hagfish]], scavenge the soft tissues at a rapid rate over a period of months, and as long as two years. This is followed by the colonization of bones and surrounding sediments (which contain organic matter) by enrichment opportunists, such as [[crustaceans]] and [[polychaetes]], throughout a period of years. Finally, sulfophilic bacteria reduce the bones releasing [[hydrogen sulphide]] enabling the growth of [[chemoautotrophic]] organisms, which in turn, support other organisms such as mussels, clams, limpets, and sea snails. This stage may last for decades and supports a rich assemblage of species, averaging 185 species per site.{{sfn|Smith et al.|2003|loc=[http://www.soest.hawaii.edu/oceanography/faculty/csmith/Files/Smith%20and%20Baco%202003.pdf The Whale Fall] pp. 311–354}}<ref>{{cite journal |last1=Fujiwara|first1=Yoshihiro|first2=Masaru|last2=Kawato|first3=Tomoko|last3=Yamamoto|first4=Toshiro|last4=Yamanaka|first5=Waka||last5=Sato-Okoshi|first6=Chikayo|last6=Noda|first7=Shinji|last7=Tsuchida1|first8=Tomoyuki|last8=Komai|first9=Sherine S.|last9=Cubelio|first10=Takenori|last10=Sasaki|first11=Karen|last11=Jacobsen|first12=Kaoru|last12=Kubokawa|first13=Katsunori|last13=Fujikura|first14=Tadashi|last14=Maruyama|first15=Yasuo|last15=Furushima|first16=Kenji|last16=Okoshi|first17=Hiroshi|last17=Miyake|first18=Masayuki|last18=Miyazaki1|first19=Yuichi|last19=Nogi|first20=Akiko|last20=Yatabe1|first21=Takashi|last21=Okutani|title=Three-year investigations into sperm whale-fall ecosystems in Japan |journal=Marine Ecology |volume=28 |issue=1 |pages=219–230|year=2007|doi=10.1111/j.1439-0485.2007.00150.x|url=http://onlinelibrary.wiley.com/doi/10.1111/j.1439-0485.2007.00150.x./pdf}}</ref> |
|||
===Keystone species=== |
|||
{{Further|Keystone species}} |
|||
[[File:BeaverDam 8409.jpg|thumb|left|[[Beaver dam]]s restrict water-flow, creating a pond]] |
|||
Sea otters are a classic example of a [[keystone species]]; their presence affects the ecosystem more profoundly than their size and numbers would suggest. They keep the population of certain [[benthic]] (sea floor) herbivores, particularly [[sea urchin]]s, in check. Sea urchins graze on the lower stems of [[kelp]], causing the kelp to drift away and die. Loss of the habitat and nutrients provided by [[kelp forest]]s leads to profound [[Cascade effect (ecology)|cascade effects]] on the marine ecosystem. North Pacific areas that do not have sea otters often turn into [[urchin barren]]s, with abundant sea urchins and no kelp forest.<ref>{{Cite journal|title = Killer Whale Predation on Sea Otters Linking Oceanic and Nearshore Ecosystems|journal = Science|year=1998|issn = 0036-8075|pmid = 9774274|pages = 473–476|volume = 282|issue = 5388|doi = 10.1126/science.282.5388.473|first = J. A.|last = Estes|first2 = M. T.|last2 = Tinker|first3 = T. M.|last3 = Williams|first4 = D. F.|last4 = Doak}}</ref> Reintroduction of sea otters to British Columbia has led to a dramatic improvement in the health of coastal ecosystems,<ref name=dfo>{{cite web|url=http://www.dfo-mpo.gc.ca/species-especes/species/species_seaOtter_e.asp|title=Aquatic Species at Risk – Species Profile – Sea Otter|publisher=Fisheries and Oceans Canada|accessdate=29 November 2007|archiveurl = https://web.archive.org/web/20071123064622/http://www.dfo-mpo.gc.ca/species-especes/species/species_seaOtter_e.asp |archivedate = November 23, 2007|deadurl=yes}}</ref> and similar changes have been observed as sea otter populations recovered in the Aleutian and Commander Islands and the [[Big Sur]] coast of California<ref name = vanblaricom33/> However, some kelp forest [[ecosystem]]s in California have also thrived without sea otters, with sea urchin populations apparently controlled by other factors.<ref name = vanblaricom33/> The role of sea otters in maintaining kelp forests has been observed to be more important in areas of open coast than in more protected bays and [[estuaries]].<ref name=vanblaricom33>VanBlaricom, p. 33</ref> |
|||
Beaver ponds have a profound effect on the surrounding ecosystem. Beaver dams hold sediment, which reduces turbidity and thereby improving overall water quality downstream. This supplies other animals with cleaner drinking water, prevents degradation of spawning grounds for fish, as well as reducing the overall temperature.<ref name=dam/><ref>{{cite web|url=https://www.pca.state.mn.us/sites/default/files/wq-iw3-21.pdf|title=Turbidity: Description, Impact on Water Quality, Sources, Measures|publisher=Minnesota Pollution Control Agency|date=March 2008|accessdate=16 June 2016}}</ref> Beaver dams also house predatory [[zooplankton]] which help break down [[detritus]] and control algae populations.<ref name=dam>{{cite journal|url=https://www.researchgate.net/publication/230462636_Downstream_effects_of_beaver_ponds_on_the_water_quality_of_New_England_first-_and_second-order_streams|first1=Leszek A.|last1=Błędzki|first2=Jill|last2=Bubier|first3=L. A.|last3=Moulton|first4=T. D.|last4=Kyker-Snowman|year=2011|title=Downstream effects of beaver ponds on the water quality of New England first‐ and second‐order streams|journal=Ecohydrology|volume=4|issue=5|pages=698–707|doi=10.1002/eco.163}}</ref> |
|||
===Apex predator=== |
|||
{{Further|Apex predator}} |
|||
An apex predator, the animal at the top of the [[food chain]], affect prey population dynamics and defense tactics (such as camoflauge).<ref>Lepak, Jesse M.; Kraft, Clifford E., Weidel, Brian C. (March 2006). [http://www.dnr.cornell.edu/cek7/Publications/Lepak_et_al_2006.pdf "Rapid food web recovery in response to removal of an introduced apex predator"] (PDF). ''Canadian Journal of Fisheries and Aquatic Sciences'' '''63''' (3): 569–575. {{ISSN|0706-652X}}. Retrieved 2010-01-25.</ref> The polar bear is the [[apex predator]] within its range, and is a [[keystone species]] for the Arctic.<ref>[http://pbsg.npolar.no/en/issues/threats/climate-change.html Climate impacts on polar bears]</ref> Several animal species, particularly [[Arctic fox]]es (''Vulpes lagopus'') and [[glaucous gull]]s (''Larus hyperboreus''), routinely scavenge polar bear kills.<ref name="behavior" /> The relationship between ringed seals and polar bears is so close that the abundance of ringed seals in some areas appears to regulate the density of polar bears, while polar bear predation in turn regulates density and reproductive success of ringed seals.<ref>{{cite book |last=Amstrup |first=Steven C. |last2=Marcot |first2=Bruce G. |last3=Douglas |first3=David C. |year=2007 |title=Forecasting the range-wide status of polar bears at selected times in the 21st Century |publication-place=Reston, Virginia |publisher=U.S. Geological Survey |url=http://www.plexusowls.com/PDFs/forecasting_polar_bears_amstrup_etal_lowres.pdf|format=PDF}}</ref> The [[evolutionary pressure]] of polar bear predation on seals probably accounts for some significant differences between Arctic and Antarctic seals. Compared to the [[Antarctica|Antarctic]], where there is no major surface predator, Arctic seals use more breathing holes per individual, appear more restless when hauled out on the ice, and rarely defecate on the ice.<ref name="behavior" /> The baby fur of most Arctic seal species is white, presumably to provide camouflage from predators, whereas Antarctic seals all have dark fur at birth.<ref name="behavior">{{cite book |last1=Stirling |first1=Ian|first2=Dan |last2=Guravich|year=1988 |title=Polar Bears |location=Ann Arbor, MI|publisher=University of Michigan Press |pages=27–28|url={{Google books|plainurl=yes|id=ViOiGWPQRjIC|page=27}}|isbn=978-0-472-10100-9|oclc=757032303}}</ref> |
|||
==Adaptations== |
==Adaptations== |
||
Revision as of 01:01, 17 June 2016


Marine mammals, which include animals such as seals, sea lions, whales, dolphins, porpoises, manatees, dugongs, sea otters, river otters, walruses, and polar bears, form a diverse group of 129 species that rely on the ocean for their existence. They do not represent a distinct biological grouping, but rather are unified by their reliance on the aquatic environment for feeding. The level of dependence on the aquatic environment for existence varies considerably with species. For example, dolphins and whales are completely dependent on the marine environment for all stages of their life, whereas seals feed in the ocean, but breed on land.
Marine mammals can be subdivided into four recognized groups; cetaceans (whales, dolphins, and porpoises) which includes the largest marine mammals, pinnipeds (seals, sea lions and walruses), sirenians (manatees and dugongs), and fissipeds, which are the group of carnivores with separate digits (the polar bear, and otters). Both cetaceans and sirenians are fully aquatic and therefore are obligate ocean dwellers. Pinnipeds are semiaquatic; they spend the majority of their time in the water, but need to return to land for important activities such as mating, breeding and molting. In contrast, both otters and the polar bear are much less adapted to aquatic living. While the number of marine mammals is small compared to those found on land, their total biomass is large. They play important roles in maintaining marine ecosystems, especially through regulation of prey populations.[1] These two factors make them an integral component of the marine environment. This is of particular concern considering 23% of marine mammal species are currently threatened.
Marine mammals were first hunted by aboriginal peoples for food and other resources. They were also the target for commercial industry, leading to a sharp decline in all populations of exploited species, such as whales and seals. Commercial hunting lead to the extinction of Steller's sea cow and the Caribbean monk seal. After commercial hunting ended, some species, such as the gray whale and northern elephant seal, have rebounded in numbers; conversely, other species, such as the North Atlantic right whale, are critically endangered. Other than hunting, marine mammals can be killed as bycatch from fisheries, where they become entangled in fixed netting and drown or starve. Increased ocean traffic causes collisions between fast ocean vessels and large marine mammals. Habitat degradation also threatens marine mammals and their ability to find and catch food. Noise pollution, for example, may adversely affect echolocating mammals, and the ongoing effects of global warming degrades arctic environments.
Taxonomy
Marine mammals form a diverse group of 129 species that rely on the ocean for their existence.[2] Of this, 23% are threatened.[3] They do not represent a distinct biological grouping, but rather are unified by their reliance on the aquatic environment for feeding.[4] The level of dependence on the aquatic environment for existence varies considerably with species. For example, dolphins and whales are completely dependent on the marine environment for all stages of their life, seals feed in the ocean but breed on land, and rhinoceroses can feed on land and in water.[4] Twenty three percent of marine mammal species are threatened.[5]
Classification of extant species
- Order Cetartiodactyla[6]
- Suborder Whippomorpha
- Family Balaenidae (right and bowhead whales), two genera and four species
- Family Neobalaenidae (pygmy right whale), one species
- Family Balaenopteridae (rorquals), two genera and eight species
- Family Eschrichtiidae (gray whale), one species
- Family Physeteridae (sperm whale), one species
- Family Kogiidae (pygmy and dwarf sperm whales), one genus and two species
- Family Monodontidae (narwhal and beluga), two genera and two species
- Family Ziphiidae (beaked whales), six genera and 21 species
- Family Delphinidae (oceanic dolphins), 17 genera and 38 species
- Family Phocoenidae (porpoises), two genera and seven species
- Family Platanistidae (South Asia river dolphin), one species
- Family Iniidae (boto) 1 species
- Family Pontoporiidae (franciscana), one species
- Family Hippopotamidae (hippopotamuses), two genera and two species
- Suborder Ruminantia
- Suborder Whippomorpha
- Order Perissodactyla
- Suborder Ceratomorpha
- Family Rhinocerotidae (rhinoceroses), one species
- Suborder Ceratomorpha
- Order Sirenia (sea cows)
- Suborder Cynodontia
- Family Trichechidae (manatees), one genus and three species
- Family Dugongidae (dugongs), one species
- Suborder Cynodontia
- Order Carnivora (carnivores):
- Suborder Caniformia
- Family Mustelidae, nine genera and 14 species
- Family Ursidae (bears), one species
- Suborder Pinnipedia (sealions, walruses, seals)
- Family Otariidae (eared seals and sea lions), seven genera and 15 species
- Family Odobenidae (walrus), one species
- Family Phocidae (true seals), 14 genera and 18 species
- Suborder Caniformia
- Order Rodentia
- Suborder Hystricomorpha
- Family Caviidae (capybara), one genera and two species
- Family Myocastoridae (nutria), one species
- Suborder Castorimorpha
- Family Castoridae (beavers), one genera and two species
- Suborder Myomorpha
- Family Cricetidae (musk rats and voles), two genera and two species
- Suborder Hystricomorpha
- Order Monotrema
- Suborder Platypoda
- Family Ornithorhynchidae (platypus), one species
- Suborder Platypoda
- Order Afrosoricida
- Suborder Tenrecomorpha
- Family Tenrecidae, one species (giant otter shrew)
- Suborder Tenrecomorpha
- Order Soricomorpha
- Family Soricidae (shrews), one species (water shrew)
- Family Talpidae (moles), one species (Russian desman)
- Order Didelphimorphia
- Family Didelphidae (opossums), two genera and two species (Lutrine opossum and Yapok)
Evolution
Mammals have returned to the water in many separate evolutionary lineages, namely: Cetacea, Sirenia, Desmostylia, Pinnipedia, Kolponomos (marine bear), Thalassocnus (aquatic sloth), and Enhydra lutris (sea otter); the eutriconodont Ichthyoconodon might have also been marine in habits. Four of these lineages are extinct (Desmostylia; Kolponomos; Thalassocnus, Dyskritodon, Ichthyoconodon).[2] Despite the diversity in morphology seen between groups, improving foraging efficiency has been the main driver in the evolution in these lineages.[7] Today, fully aquatic marine mammals belong to one of two orders: Cetartiodactyla or Sirenia

Based on molecular and morphological research, the cetaceans genetically and morphologically fall firmly within the Artiodactyla (even-toed ungulates).[8][9] The term Cetartiodactyla reflects the idea that whales evolved within the ungulates. The term was coined by merging the name for the two orders, Cetacea and Artiodactyla, into a single word. Under this definition, the closest living land relative of the whales and dolphins is thought to be the hippopotamuses. Use of the order Cetartiodactyla, instead of Cetacea with parvorders Odontoceti and Mysticeti, is favored by most evolutionary mammalogists working with molecular data[10][11][12][13] and is supported the IUCN Cetacean Specialist Group[14] and by Taxonomy Committee[6] of the Society for Marine Mammalogy, the largest international association of marine mammal scientists in the world. Some others, including many marine mammalogists and paleontologists, favor retention of order Cetacea with the two suborders in the interest of taxonomic stability.
Fossil evidence indicates the sea otter (Enhydra) lineage became isolated in the North Pacific approximately 2 Mya, giving rise to the now-extinct Enhydra macrodonta and the modern sea otter, Enhydra lutris. The sea otter evolved initially in northern Hokkaidō and Russia, and then spread east to the Aleutian Islands, mainland Alaska, and down the North American coast. In comparison to cetaceans, sirenians, and pinnipeds, which entered the water approximately 50, 40, and 20 Mya, respectively, the sea otter is a relative newcomer to marine life. In some respects, though, the sea otter is more fully adapted to water than pinnipeds, which must haul out on land or ice to give birth.[15] An extinct genus, Satherium, is believed to be ancestral to the South American variety, having migrated to the New World during the Pliocene or early Pleistocene.[16] The South American continent houses the Lontra genus of otters: the giant otter, the neotropical river otter, the southern river otter, and the marine otter.[17] The smooth-coated otter (Lutrogale perspicillata) of Asia may be its closest extant relative; similar behaviour, vocalizations, and skull morphology have been noted.[16]

The first appearance of sirenians in the fossil record was during the early Eocene, and by the late Eocene, sirenians had significantly diversified. Inhabitants of rivers, estuaries, and nearshore marine waters, they were able to spread rapidly. The most primitive sirenian, Prorastomus, was found in Jamaica, unlike other marine mammals which originated from the Old World (such as cetaceans[18]). The first known quadrupedal sirenian was Pezosiren from the early Eocene.[19] The earliest known sea cows, of the families Prorastomidae and Protosirenidae, are both confined to the Eocene, and were pig-sized, four-legged, amphibious creatures.[20] The first members of Dugongidae appeared by the end of the Eocene.[21] At this point, sea cows were fully aquatic.[20]
The first marine mammals appeared in the Jurassic. The teeth of Castorocauda, a presumably beaver-like mammal, are different in many ways from all other docodonts, presumably due to a difference in diet. Most docodonts had teeth specialized for an omnivorous diet. The teeth of Castorocauda suggest that the animal was a piscivore, feeding on fish and small invertebrates. The first two molars had cusps in a straight row, eliminating the grinding function suggesting that they were strictly for gripping and not for chewing. This feature of three cusps in a row is similar to the ancestral condition in mammal relatives (as seen in triconodonts), but is almost certainly a derived character in Castorocauda. These first molars were also recurved in a manner designed to hold slippery prey once grasped. These teeth are very similar to the teeth seen in mesonychids, an extinct group of semi-aquatic carnivorous ungulates, and resemble, to a lesser degree, the teeth of seals.[22]
Ecology
Distribution and habitat

Marine mammals are widely distributed throughout the globe, but their distribution is patchy and coincides with the productivity of the oceans.[23] Species richness peaks at around 40° latitude, both north and south. This corresponds to the highest levels of primary production around North and South America, Africa, Asia and Australia. Total species range is highly variable for marine mammal species. On average most marine mammals have ranges which are equivalent or smaller than one-fifth of the Indian Ocean.[5] The variation observed in range size is a result of the different ecological requirements of each species and their ability to cope with a broad range of environmental conditions. There is a high degree of overlap between marine mammal species richness and areas of human impact on the environment which is of concern.[1]
Most marine mammals, such as seals and sea otters, inhabit the coast. Seals, however, also use a number of terrestrial habitats, both continental and island. In temperate and tropical areas, they haul-out on to sandy and pebble beaches, rocky shores, shoals, mud flats, tide pools and in sea caves. Some species also rest on man-made structures, like piers, jetties, buoys and oil platforms. Seals may move further inland and rest in sand dunes or vegetation, and may even climb cliffs.[24]: 96 Most cetaceans live in the open ocean, and species like the sperm whale may dive to depths of −1,000 to −2,500 feet (−300 to −760 m) in search of food.[25] Sirenians live in shallow coastal waters, usually living 30 feet (9.1 m) below sea level. However, they have been known to dive to −120 feet (−37 m) to forage deep-water seagrasses.[26] Freshwater variety, generally wade in the shallow parts of lakes, with a few exceptions, and are bottom-feeders.[27]
Whale pump
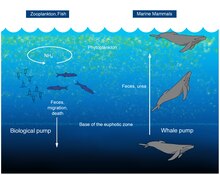
A 2010 study considered whales to be a positive influence to the productivity of ocean fisheries, in what has been termed a "whale pump". Whales carry nutrients such as nitrogen from the depths back to the surface. This functions as an upward biological pump, reversing an earlier presumption that whales accelerate the loss of nutrients to the bottom. This nitrogen input in the Gulf of Maine is "more than the input of all rivers combined" emptying into the gulf, some 23,000 metric tons (25,000 short tons) each year.[28] Whales defecate at the ocean's surface; their excrement is important for fisheries because it is rich in iron and nitrogen. The whale faeces are liquid and instead of sinking, they stay at the surface where phytoplankton feed off it.[28][29]
Upon death, whale carcasses fall to the deep ocean and provide a substantial habitat for marine life. Evidence of whale falls in present-day and fossil records shows that deep sea whale falls support a rich assemblage of creatures, with a global diversity of 407 species, comparable to other neritic biodiversity hotspots, such as cold seeps and hydrothermal vents.[30] Deterioration of whale carcasses happens though a series of three stages. Initially, moving organisms such as sharks and hagfish, scavenge the soft tissues at a rapid rate over a period of months, and as long as two years. This is followed by the colonization of bones and surrounding sediments (which contain organic matter) by enrichment opportunists, such as crustaceans and polychaetes, throughout a period of years. Finally, sulfophilic bacteria reduce the bones releasing hydrogen sulphide enabling the growth of chemoautotrophic organisms, which in turn, support other organisms such as mussels, clams, limpets, and sea snails. This stage may last for decades and supports a rich assemblage of species, averaging 185 species per site.[31][32]
Keystone species

Sea otters are a classic example of a keystone species; their presence affects the ecosystem more profoundly than their size and numbers would suggest. They keep the population of certain benthic (sea floor) herbivores, particularly sea urchins, in check. Sea urchins graze on the lower stems of kelp, causing the kelp to drift away and die. Loss of the habitat and nutrients provided by kelp forests leads to profound cascade effects on the marine ecosystem. North Pacific areas that do not have sea otters often turn into urchin barrens, with abundant sea urchins and no kelp forest.[33] Reintroduction of sea otters to British Columbia has led to a dramatic improvement in the health of coastal ecosystems,[34] and similar changes have been observed as sea otter populations recovered in the Aleutian and Commander Islands and the Big Sur coast of California[35] However, some kelp forest ecosystems in California have also thrived without sea otters, with sea urchin populations apparently controlled by other factors.[35] The role of sea otters in maintaining kelp forests has been observed to be more important in areas of open coast than in more protected bays and estuaries.[35]
Beaver ponds have a profound effect on the surrounding ecosystem. Beaver dams hold sediment, which reduces turbidity and thereby improving overall water quality downstream. This supplies other animals with cleaner drinking water, prevents degradation of spawning grounds for fish, as well as reducing the overall temperature.[36][37] Beaver dams also house predatory zooplankton which help break down detritus and control algae populations.[36]
Apex predator
An apex predator, the animal at the top of the food chain, affect prey population dynamics and defense tactics (such as camoflauge).[38] The polar bear is the apex predator within its range, and is a keystone species for the Arctic.[39] Several animal species, particularly Arctic foxes (Vulpes lagopus) and glaucous gulls (Larus hyperboreus), routinely scavenge polar bear kills.[40] The relationship between ringed seals and polar bears is so close that the abundance of ringed seals in some areas appears to regulate the density of polar bears, while polar bear predation in turn regulates density and reproductive success of ringed seals.[41] The evolutionary pressure of polar bear predation on seals probably accounts for some significant differences between Arctic and Antarctic seals. Compared to the Antarctic, where there is no major surface predator, Arctic seals use more breathing holes per individual, appear more restless when hauled out on the ice, and rarely defecate on the ice.[40] The baby fur of most Arctic seal species is white, presumably to provide camouflage from predators, whereas Antarctic seals all have dark fur at birth.[40]
Adaptations

Saltwater
Marine mammals have a number of physiological and anatomical features to overcome the unique challenges associated with aquatic living. Some of these features are very species specific. Marine mammals have developed a number of features for efficient locomotion such as torpedo shaped bodies to reduce drag; modified limbs for propulsion and steering; tail flukes and dorsal fins for propulsion and balance.[23] Marine mammals are adept at thermoregulation using dense fur or blubber to reduce heat loss; as well as circulatory adjustments to conserve their body temperature (counter-current heat exchangers); torpedo shaped bodies, reduced appendages, and large size to prevent heat loss.[23]
Most marine mammals are hypoosmotic and as a result they are constantly losing water to the surrounding environment. They have evolved a number of mechanisms to overcome this, but most retain their water by using highly efficient kidneys, that can concentrate urine.[23] Marine mammals are able to dive for long periods of time. Both pinnipeds and cetaceans have large and complex blood vessel systems which serve to store oxygen to support deep diving. Other important reservoirs include muscles, blood, and the spleen which all have the capacity to hold a high concentration of oxygen. Other features include bradycardia (reduced heart rate), and vasoconstriction (shunts most of the oxygen to vital organs such as the brain and heart) also assist with extended diving and oxygen deprivation.[23]
If oxygen is depleted, marine mammals can access substantial reservoirs of glycogen that support anaerobic glycolysis of the cells involved during conditions of systemic hypoxia associated with prolonged submersion.[42][43][44] Sound travels differently through water therefore marine mammals have developed a number of ways to ensure effective communication, prey capture, and predator detection.[45] The most notable adaptation is the development of echolocation in whales and dolphins.[23] Marine mammals have evolved a number features for feeding, which are mainly seen in their dentition. For example, the cheek teeth of pinniped and odontocetes are designed specifically to capture fish and squid. In contrast, Mysticetes have evolved baleen plates to filter feed plankton and small fish from the water.[23]
Freshwater

Polar bears, otters, seals, sea lions, and beavers have fur, one of the defining mammalian features, that is oily and waterproof in order to trap air to provide insulation. In contrast, other marine mammals – such as whales, dolphins, porpoises, manatees, dugongs, walruses, and hippopotamuses – have lost long fur in favor of a thick, dense epidermis and a thickened fat layer (blubber) in response to hydrodynamic requirements.[27]
Wading and bottom-feeding animals (such as moose and manatee) need to be heavier than water in order to keep contact with the floor or to stay submerged. Surface-living animals (such as river otters) need the opposite, and free-swimming animals living in open waters (such as dolphins) need to be neutrally buoyant in order to be able to swim up and down. Typically, thick and dense bone is found in bottom feeders and low bone density is associated with mammals living in deep water.[27]
Some marine mammals have retained four weight-bearing limbs (such as polar bears, beaver, otter, muskrat) and can walk on land like fully terrestrial animals. The long and thin legs of a moose limit exposure to and friction from water in contrast to hippopotamuses who keep most of their body submerged and have short and thick legs. The semiaquatic pygmy hippopotamus can walk quickly on a muddy underwater surface thanks to robust muscles and because all toes are weight-bearing.[27]
Diet
Carnivores

All cetaceans are carnivorous and predatory. Toothed whales mostly feed on fish and cephalopods, followed by crustaceans and bivalves. Some may forage with other kinds of animals, such as other species of whales or certain species of pinnipeds.[24]: 169 [46] One common feeding method is herding, where a pod squeezes a school of fish into a small volume, known as a bait ball. Individual members then take turns plowing through the ball, feeding on the stunned fish.[47] Coralling is a method where dolphins chase fish into shallow water to catch them more easily.[47] Killer whales and bottlenose dolphins have also been known to drive their prey onto a beach to feed on it.[48][49] Other whales with a blunt snout and reduced dentition rely on suction feeding.[50]
Baleen whales use their baleen plates to sieve plankton, among others, out of the water; there are two types of methods: lunge-feeding and gulp-feeding. Lunge-feeders expand the volume of their jaw to a volume bigger than the original volume of the whale itself by inflating their mouth. This causes grooves on their throat to expand, increasing the amount of water the mouth can store.[51][52] They ram a baitball at high speeds in order to feed, but this is only energy-effective when used against a large baitball.[53] Gulp-feeders swim with an open mouth, filling it with water and prey. Prey must occur in sufficient numbers to trigger the whale's interest, be within a certain size range so that the baleen plates can filter it, and be slow enough so that it cannot escape.[54]

Otters are the only marine animal capable of lifting and turning over rocks, which it often does with its front paws when searching for prey.[55] The sea otter may pluck snails and other organisms from kelp and dig deep into underwater mud for clams.[55] It is the only marine mammal that catches fish with its forepaws rather than with its teeth.[56] Under each foreleg, the sea otter has a loose pouch of skin that extends across the chest which they use to store collected food to bring to the surface. This pouch also holds a rock that is used to break open shellfish and clams.[57] There, the sea otter eats while floating on its back, using its forepaws to tear food apart and bring it to its mouth.[58][59]
Pinnipeds mostly feed on fish and cephalopods, followed by crustaceans and bivalves, and then zooplankton and warm-blooded prey (like sea birds).[24]: 145 Most species are generalist feeders, but a few are specialists.[60] They typical when hunt non-schooling fish, slow-moving or immobile invertebrates or endothermic prey in groups. Solitary foraging species usually exploit coastal waters, bays and rivers. When large schools of fish or squid are available, pinnipeds hunt cooperatively in large groups, locating and herding their prey. Some species, such as California and South American sea lions, may forage with cetaceans and sea birds.[24]: 168
The polar bear is the most carnivorous bear, and its diet primarily consists of ringed (Pusa hispida) and bearded seals (Erignathus barbatus).[61] Polar bears hunt primarily at the interface between ice, water, and air; they only rarely catch seals on land or in open water.[62] The polar bear's most common hunting method is still-hunting:[63] The bear locates a seal breathing hole using its sense of smell, and crouches nearby for a seal to appear. When the seal exhales, the bear smells its breath, reaches into the hole with a forepaw, and drags it out onto the ice. The polar bear also hunts by stalking seals resting on the ice. Upon spotting a seal, it walks to within 90 m (100 yd), and then crouches. If the seal does not notice, the bear creeps to within 9 to 12 m (30 to 40 ft) of the seal and then suddenly rushes to attack.[64] A third hunting method is to raid the birth lairs that female seals create in the snow.[63] They may also feed on fish.[65]
Generally, all aquatic desmans, shrews, and voles make quick dives and catch small fish and invertebrates. The giant otter shrew, for example, makes quick dives that last for seconds and grabs small crabs (usually no bigger than 2.8 inches (7 cm) across).[66] The Lutrine opossum is the most carnivorous opossum, usually consuming small birds, rodents, and invertebrates.[67] Water voles mainly eat grass and plants near the water and at times, they will also consume fruits, bulbs, twigs, buds, and roots. However, a population of water voles living in Wiltshire and Lincolnshire, England have started eating frogs' legs and discarding the bodies.[68]
Herbivores

Sirenians are referred to as "sea cows" because their diet consists mainly of sea-grass. When eating they ingest the whole plant, including the roots, although when this is impossible they will feed on just the leaves.[69] A wide variety of seagrass has been found in dugong stomach contents, and evidence exists they will eat algae when seagrass is scarce.[70]
Beavers are herbivores, and prefer the wood of quaking aspen, cottonwood, willow, alder, birch, maple and cherry trees. They also eat sedges, pondweed, and water lilies.[71] Beavers do not hibernate, but rather they store sticks and logs in a pile in their ponds, eating the underbark. The dams they build flood areas of surrounding forest, giving the beaver safe access to an important food supply, which is the leaves, buds, and inner bark of growing trees. They prefer aspen and poplar, but will also take birch, maple, willow, alder, black cherry, red oak, beech, ash, hornbeam and occasionally pine and spruce.[72] They will also eat cattails, water lilies and other aquatic vegetation, especially in the early spring.[73]
Hippopotamuses leave the water at dusk and travel inland, sometimes up to 10 km (6 mi),[74] to graze on short grasses, their main source of food. They spend four to five hours grazing and can consume 68 kg (150 lb) of grass each night.[75] Like almost any herbivore, they consume other plants if presented with them, but their diet consists almost entirely of grass, with only minimal consumption of aquatic plants.[76] The pygmy hippopotamus emerges from the water at dusk to feed. It relies on game trails to travel through dense forest vegetation. It marks trails by vigorously waving its tail while defecating to further spread its feces. The pygmy hippo spends about six hours a day foraging for food, and they do not eat aquatic vegetation to a significant extent and rarely eat grass because it is uncommon in the thick forests they inhabit. The bulk of a pygmy hippo's diet consists of ferns, broad-leaved plants and fruits that have fallen to the forest floor. The wide variety of plants pygmy hippos have been observed eating suggests that they will eat any plants available. This diet is of higher quality than that of the common hippopotamus.[77][78]
Indian rhinoceros are grazers. Their diets consist almost entirely of grasses, but they also eat leaves, branches of shrubs and trees, fruits, and submerged and floating aquatic plants. They feed in the mornings and evenings. They use their prehensile lips to grasp grass stems, bend the stem down, bite off the top, and then eat the grass. They tackle very tall grasses or saplings by walking over the plant, with legs on both sides and using the weight of their bodies to push the end of the plant down to the level of the mouth.[79]
A moose's diet often depends on its location, but they seem to prefer the new growths from deciduous trees with a high sugar content, such as white birch, trembling aspen and striped maple, among many others.[80] They also eat many aquatic plants such as lilies and water milfoil.[81] To reach high branches, a moose may bend small saplings down, using its prehensile lip, mouth or body. For larger trees a moose may stand erect and walk upright on its hind legs, allowing it to reach plants 14.0 feet (4.26 m) off the ground.[82][83] Moose are excellent swimmers and are known to wade into water to eat aquatic plants. Moose are thus attracted to marshes and river banks during warmer months as both provide suitable vegetation to eat and water to wet themselves in. Moose have been known to dive underwater to reach plants on lake bottoms, and the complex snout may assist the moose in this type of feeding. Moose are the only deer that are capable of feeding underwater.[84]
Threats
Exploitation

Marine mammals were hunted by coastal aboriginal humans historically for food and other resources. These subsistence hunts still occur in Canada, Greenland, Indonesia, Russia, the United States, and several nations in the Caribbean. The effects of these are only localized, as hunting efforts were on a relatively small scale.[23] Commercial hunting took this to a much greater scale and marine mammals were heavily exploited. This led to the extinction of the Steller's sea cow and the Caribbean monk seal.[23] Today, populations of species that were historically hunted, such as blue whales (Balaenoptera musculus musculus and B. m. brevicauda), and the North Pacific right whale (Eubalaena japonica), are much lower compared to their pre-whaling levels.[85] Because whales generally have slow growth rates, are slow to reach sexual maturity, and have a low reproductive output, population recovery has been very slow.[45]
A number of whales are still subject to direct hunting, despite the 1986 moratorium ban on whaling set under the terms of the International Whaling Commission (IWC). There are only two nations remaining which sanction commercial whaling: Norway, where several hundred common minke whales are harvested each year; and Iceland, where quotas of 150 fin whales and 100 minke whales per year are set.[86][87] Japan also harvests several hundred Antarctic and North Pacific minke whales each year for scientific research (in accordance with the moratorium).[85] However, the illegal trade of whale and dolphin meat is a significant market in some countries.[88]
Commercial sealing was historically just as important as the whaling industry. Exploited species included harp seals, hooded seals, Caspian seals, elephant seals, walruses and all species of fur seal.[89] The scale of seal harvesting decreased substantially after the 1960s,[90] after the Canadian government reduced the length of the hunting season and implemented measures to protect adult females.[91] Several species that were commercially exploited have rebounded in numbers; for example, Antarctic fur seals may be as numerous as they were prior to harvesting. The northern elephant seal was hunted to near extinction in the late 19th century, with only a small population remaining on Guadalupe Island. It has since recolonized much of its historic range, but has a population bottleneck.[89] Conversely, the Mediterranean monk seal was extirpated from much of its former range, which stretched from the Mediterranean to the Black Sea and northwest Africa, and only remains in the northeastern Mediterranean and some parts of northwest Africa.[92]
Ocean traffic and fisheries

By-catch is the incidental capture of non-target species in fisheries. Fixed and drift gill nets cause the highest mortality levels for both cetaceans and pinnipeds, however, entanglements in long lines, mid-water trawls, and both trap and pot lines are also common.[93] Tuna seines are particularly problematic for entanglement by dolphins.[94] By-catch affects all cetaceans, both small and big, in all habitat types. However, smaller cetaceans and pinnipeds are most vulnerable as their size means that escape once they are entangled is highly unlikely and they frequently drown.[85] While larger cetaceans are capable of dragging nets with them, the nets sometimes remain tightly attached to the individual and can impede the animal from feeding sometimes leading to starvation.[85] Abandoned or lost nets and lines cause mortality through ingestion or entanglement.[95] Marine mammals also get entangled in aquaculture nets, however, these are rare events and not prevalent enough to impact populations.[96]
Vessel strikes cause death for a number of marine mammals, especially whales.[85] In particular, fast commercial vessels such as container ships can cause major injuries or death when they collide with marine mammals. Collisions occur both with large commercial vessels and recreational boats and cause injury to whales or smaller cetaceans. The critically endangered North Atlantic right whale is particularly affected by vessel strikes.[97] Tourism boats designed for whale and dolphin watching can also negatively impact on marine mammals by interfering with their natural behavior.[98]
The fishery industry not only threatens marine mammals through by-catch, but also through competition for food. Large scale fisheries have led to the depletion of fish stocks that are important prey species for marine mammals. Pinnipeds have been especially affected by the direct loss of food supplies and in some cases the harvesting of fish has led to food shortages or dietary deficiencies,[99] starvation of young, and reduced recruitment into the population.[100] As the fish stocks have been depleted, the competition between marine mammals and fisheries has sometimes led to conflict. Large-scale culling of populations of marine mammals by commercial fishers has been initiated in a number of areas in order to protect fish stocks for human consumption.[101]
Shellfish aquaculture takes up space so in effect creates competition for space. However, there is little direct competition for aquaculture shellfish harvest.[96] On the other hand, marine mammals regularly take finfish from farms, which creates significant problems for marine farmers. While there are usually legal mechanisms designed to deter marine mammals, such as anti-predator nets or harassment devices, individuals are often illegally shot.[96]
Habitat loss and degradation

Habitat degradation is caused by a number of human activities. Marine mammals that live in coastal environments are most likely to be affected by habitat degradation and loss. Developments such as sewage marine outfalls, moorings, dredging, blasting, dumping, port construction, hydroelectric projects, and aquaculture both degrade the environment and take up valuable habitat.[45] For example, extensive shellfish aquaculture takes up valuable space used by coastal marine mammals for important activities such as breeding, foraging and resting.[96]
Contaminants that are discharged into the marine environment accumulate in the bodies of marine mammals when they are stored unintentionally in their blubber along with energy.[45] Contaminants that are found in the tissues of marine mammals include heavy metals, such as mercury and lead, but also organochlorides and polycyclic aromatic hydrocarbons.[45] For example, these can cause disruptive effects on endocrine systems;[95] impair the reproductive system, and lower the immune system of individuals, leading to a higher number of deaths.[45] Other pollutants such as oil, plastic debris and sewage threaten the livelihood of marine mammals.[102]
Noise pollution from anthropogenic activities is another major concern for marine mammals. This is a problem because underwater noise pollution interferes with the abilities of some marine mammals to communicate, and locate both predators and prey.[103] Underwater explosions are used for a variety of purposes including military activities, construction and oceanographic or geophysical research. They can cause injuries such as hemorrhaging of the lungs, and contusion and ulceration of the gastrointestinal tract.[85] Underwater noise is generated from shipping, the oil and gas industry, research, and military use of sonar and oceanographic acoustic experimentation. Acoustic harassment devices and acoustic deterrent devices used by aquaculture facilities to scare away marine mammals emit loud and noxious underwater sounds.[96]
Two changes to the global atmosphere due to anthropogenic activity threaten marine mammals. The first is increases in ultraviolet radiation due to ozone depletion, and this mainly affects the Antarctic and other areas of the southern hemisphere.[45] An increase in ultraviolet radiation has the capacity to decrease phytoplankton abundance, which forms the basis of the food chain in the ocean.[104] The second effect of global climate change is global warming due to increased carbon dioxide levels in the atmosphere. Raised sea levels, sea temperature and changed currents are expected to affect marine mammals by altering the distribution of important prey species, and changing the suitability of breeding sites and migratory routes.[105] The Arctic food chain would be disrupted by the near extinction or migration of polar bears. Arctic sea ice is the polar bear’s habitat. It has been declining at a rate of 13% per decade because the temperature is rising at twice the rate of the rest of the world.[106] By the year 2050, up to two-thirds of the world's polar bears may vanish if the sea ice continues to melt at its current rate.[107]
See also
- Aquatic mammal
- Aquatic animal
- List of semiaquatic tetrapods
- Marine mammals as food
- U.S. Navy Marine Mammal Program
References
- ^ a b Kaschner, K.; Tittensor, D. P.; Ready, J.; Gerrodette, T.; Worm, B. (2011). "Current and Future Patterns of Global Marine Mammal Biodiversity". PLoS ONE. 6 (5): e19653. doi:10.1371/journal.pone.0019653.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Pompa, S.; Ehrlich, P. R.; Ceballos, G. (2011-08-16). "Global distribution and conservation of marine mammals". Proceedings of the National Academy of Sciences. 108 (33): 13600–13605. doi:10.1073/pnas.1101525108.
- ^ Schipper, J.; Chanson, J. S.; Chiozza, F.; Cox, N. A. (2008). "The status of the world's land and marine mammals: diversity, threat, and knowledge". Science. 322 (5899): 225–230. doi:10.1126/science.1165115. PMID 18845749.
- ^ a b Jefferson, T. A.; Webber, M. A.; Pitman, R. L. (2009). Marine Mammals of the World A Comprehensive Guide to their Identification (1 ed.). London: Academic Press. pp. 7–16. ISBN 978-0-12-383853-7. OCLC 326418543.
- ^ a b Schipper, J.; Chanson, J. S.; Chiozza, F.; Cox, N. A.; Hoffmann, M.; Katariya, V.; Lamoreux, J.; Rodrigues, A. S. L.; Stuart, S. N.; Temple, H. J.; Baillie, J.; Boitani, L.; Lacher, T. E.; Mittermeier, R. A.; Smith, A. T.; Absolon, D.; Aguiar, J. M.; Amori, G.; Bakkour, N.; Baldi, R.; Berridge, R. J.; Bielby, J.; Black, P. A.; Blanc, J. J.; Brooks, T. M.; Burton, J. A.; Butynski, T. M.; Catullo, G.; Chapman, R.; et al. (2008). "The Status of the World's Land and Marine Mammals: Diversity, Threat, and Knowledge" (PDF). Science. 322 (5899): 225–30. doi:10.1126/science.1165115. PMID 18845749.
- ^ a b "The Society for Marine Mammalogy's Taxonomy Committee List of Species and subspecies".
- ^ Uhen, M. D. (2007). "Evolution of marine mammals: Back to the sea after 300 million years". The Anatomical Record. 290 (6): 514–22. doi:10.1002/ar.20545. PMID 17516441.
- ^ Geisler, Jonathan H.; Uden, Mark D. (2005). "Phylogenetic Relationships of Extinct Cetartiodactyls: Results of Simultaneous Analyses of Molecular, Morphological, and Stratigraphic Data". Journal of Mammalian Evolution. 12 (1–2): 145–160. doi:10.1007/s10914-005-4963-8.
- ^ Graur, D.; Higgins, G. (1994). "Molecular evidence for the inclusion of cetaceans within the order Artiodactyla" (PDF). Molecular Biology and Evolution. 11 (3): 357–364. PMID 8015431.
- ^ Agnarsson, I.; May-Collado, LJ. (2008). "The phylogeny of Cetartiodactyla: the importance of dense taxon sampling, missing data, and the remarkable promise of cytochrome b to provide reliable species-level phylogenies". Mol Phylogenet Evol. 48 (3): 964–985. doi:10.1016/j.ympev.2008.05.046. PMID 18590827.
- ^ Price, SA.; Bininda-Emonds, OR.; Gittleman, JL. (2005). "A complete phylogeny of the whales, dolphins and even-toed hoofed mammals – Cetartiodactyla". Biol Rev Camb Philos Soc. 80 (3): 445–473. doi:10.1017/s1464793105006743. PMID 16094808.
- ^ Montgelard, C.; Catzeflis, FM.; Douzery, E. (1997). "Phylogenetic relationships of artiodactyls and cetaceans as deduced from the comparison of cytochrome b and 12S RNA mitochondrial sequences". Molecular Biology and Evolution. 14 (5): 550–559. doi:10.1093/oxfordjournals.molbev.a025792. PMID 9159933.
- ^ Spaulding, M.; O'Leary, MA.; Gatesy, J. (2009). "Relationships of Cetacea -Artiodactyla- Among Mammals: Increased Taxon Sampling Alters Interpretations of Key Fossils and Character Evolution". PLoS ONE. 4 (9): e7062. Bibcode:2009PLoSO...4.7062S. doi:10.1371/journal.pone.0007062. PMC 2740860. PMID 19774069.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cetacean Species and Taxonomy. iucn-csg.org
- ^ Love, John A. (1992). Sea Otters. Golden, Colorado: Fulcrum Publishing. pp. 4–16. ISBN 1-55591-123-4. OCLC 25747993.
- ^ a b Koepfli, K.-P; Wayne, R.K. (December 1998). "Phylogenetic relationships of otters (Carnivora: Mustelidae) based on mitochondrial cytochrome b sequences". Journal of Zoology. 246 (4): 401–416. doi:10.1111/j.1469-7998.1998.tb00172.x.
- ^ Foster-Turley, Pat; Macdonald, Sheila; Mason, Chris (eds.) (1990). "Otters: An Action Plan for their Conservation". IUCN/SSC Otter Specialist Group. International Conservation Union: Sections 2 and 12. Retrieved 2007-11-21.
{{cite journal}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Thewissen, J. G. M.; Bajpai, Sunil (2001). "Whale Origins as a Poster Child for Macroevolution". BioScience. 51 (12): 1037–1049. doi:10.1641/0006-3568(2001)051[1037:WOAAPC]2.0.C.
- ^ Domning DP (2001). "The Earliest Known Fully Quadrupedal Sirenian". Nature. 413 (6856): 625–627. doi:10.1038/35098072. PMID 11675784.
- ^ a b Prins, Herbert H. T.; Gordon, Iain J., eds. (2014). "The Biological Invasion of Sirenia into Australasia". Invasion Biology and Ecological Theory. Cambridge: Cambridge University Press. p. 123. ISBN 978-1-107-03581-2. OCLC 850909221.
- ^ Samonds, K. E.; Zalmout, I. S.; Irwin, M. T.; Krause, D. W.; Rogers, R. R.; Raharivony, L. L. (2009). "Eotheroides lambondrano, new Middle Eocene seacow (Mammalia, Sirenia) from the Mahajanga Basin, Northwestern Madagascar". Journal of Vertebrate Paleontology. 29 (4): 1233–1243. doi:10.1671/039.029.0417.
- ^ Ji, Qiang; Luo, Zhe-Xi; Yuan, Chong-Xi; Tabrum, Alan R. (2006). "A Swimming Mammaliaform from the Middle Jurassic and Ecomorphological Diversification of Early Mammals". Science. 311 (5764): 1123–1127. doi:10.1126/science.1123026. PMID 16497926.
- ^ a b c d e f g h i Berta, A; Sumich, J. L. (1999). Marine Mammals: Evolutionary Biology. San Diego: Academic Press. ISBN 978-0-12-093225-2. OCLC 42467530.
- ^ a b c d Riedman, M. (1990). The Pinnipeds: Seals, Sea Lions, and Walruses. Los Angeles: University of California Press. ISBN 978-0-520-06497-3. OCLC 19511610.
- ^ Whitehead, H. (2003). Sperm Whales: Social Evolution in the Ocean. Chicago: University of Chicago Press. p. 79. ISBN 978-0-226-89518-5. OCLC 51242162.
- ^ Marsh, H.; Eros, Carole; Hugues, Joanna; Penrose, Helen (2002). Dugong: status reports and action plans for countries and territories (PDF). International Union for Conservation of Nature. ISBN 978-92-807-2130-0. OCLC 51040880.
- ^ a b c d Reidenberg, Joy S. (2007). "Anatomical Adaptations of Aquatic Mammals". The Anatomical Record. 290 (6): 507–513. doi:10.1002/ar.20541. OCLC 255630658. PMID 17516440.
- ^ a b c Roman, J.; McCarthy, J. J. (2010). Roopnarine, Peter (ed.). "The Whale Pump: Marine Mammals Enhance Primary Productivity in a Coastal Basin". PLoS ONE. 5 (10). doi:10.1371/journal.pone.0013255.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Roman, Joe; Estes, James A.; Morissette, Lyne; Smith, Craig; Costa, Daniel; McCarthy, James; Nation, J.B.; Nicol, Stephen; Pershing, Andrew; Smetacek, Victor (2014). "Whales as marine ecosystem engineers". Frontiers in Ecology and the Environment. 12 (7): 377–385. doi:10.1890/130220.
- ^ Smith, Craig R.; Baco, Amy R. (2003). "Ecology of Whale Falls at the Deep-Sea Floor" (PDF). Oceanography and Marine Biology: An Annual Review. 41: 311–354.
- ^ Smith et al. 2003, The Whale Fall pp. 311–354.
- ^ Fujiwara, Yoshihiro; Kawato, Masaru; Yamamoto, Tomoko; Yamanaka, Toshiro; Sato-Okoshi, Waka; Noda, Chikayo; Tsuchida1, Shinji; Komai, Tomoyuki; Cubelio, Sherine S.; Sasaki, Takenori; Jacobsen, Karen; Kubokawa, Kaoru; Fujikura, Katsunori; Maruyama, Tadashi; Furushima, Yasuo; Okoshi, Kenji; Miyake, Hiroshi; Miyazaki1, Masayuki; Nogi, Yuichi; Yatabe1, Akiko; Okutani, Takashi (2007). "Three-year investigations into sperm whale-fall ecosystems in Japan". Marine Ecology. 28 (1): 219–230. doi:10.1111/j.1439-0485.2007.00150.x.
{{cite journal}}: Cite has empty unknown parameter:|1=(help)CS1 maint: numeric names: authors list (link) - ^ Estes, J. A.; Tinker, M. T.; Williams, T. M.; Doak, D. F. (1998). "Killer Whale Predation on Sea Otters Linking Oceanic and Nearshore Ecosystems". Science. 282 (5388): 473–476. doi:10.1126/science.282.5388.473. ISSN 0036-8075. PMID 9774274.
- ^ "Aquatic Species at Risk – Species Profile – Sea Otter". Fisheries and Oceans Canada. Archived from the original on November 23, 2007. Retrieved 29 November 2007.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c VanBlaricom, p. 33
- ^ a b Błędzki, Leszek A.; Bubier, Jill; Moulton, L. A.; Kyker-Snowman, T. D. (2011). "Downstream effects of beaver ponds on the water quality of New England first‐ and second‐order streams". Ecohydrology. 4 (5): 698–707. doi:10.1002/eco.163.
- ^ "Turbidity: Description, Impact on Water Quality, Sources, Measures" (PDF). Minnesota Pollution Control Agency. March 2008. Retrieved 16 June 2016.
- ^ Lepak, Jesse M.; Kraft, Clifford E., Weidel, Brian C. (March 2006). "Rapid food web recovery in response to removal of an introduced apex predator" (PDF). Canadian Journal of Fisheries and Aquatic Sciences 63 (3): 569–575. ISSN 0706-652X. Retrieved 2010-01-25.
- ^ Climate impacts on polar bears
- ^ a b c Stirling, Ian; Guravich, Dan (1988). Polar Bears. Ann Arbor, MI: University of Michigan Press. pp. 27–28. ISBN 978-0-472-10100-9. OCLC 757032303.
- ^ Amstrup, Steven C.; Marcot, Bruce G.; Douglas, David C. (2007). Forecasting the range-wide status of polar bears at selected times in the 21st Century (PDF). Reston, Virginia: U.S. Geological Survey.
- ^ Pfeiffer, Carl J. (1997). "Renal cellular and tissue specializations in the bottlenose dolphin (Tursiops truncatus) and beluga whale (Delphinapterus leucas)" (PDF). Aquatic Mammals. 23 (2): 75–84. Retrieved 2014-04-25.
- ^ Lockyer, Christina (1991). "Body composition of the sperm whale, Physeter cation, with special reference to the possible functions of fat depots" (PDF). Journal of the Marine Research Institute. 12 (2). ISSN 0484-9019. Retrieved 2014-04-25.
The significant levels of carbohydrate, probably mostly in the form of glycogen, in both blubber and muscle, may represent an instant form of energy for diving via anaerobic glycolysis.
- ^ Hochachka, P.; Storey, K. (1975). "Metabolic consequences of diving in animals and man". Science. 187 (4177): 613–621. Bibcode:1975Sci...187..613H. doi:10.1126/science.163485. ISSN 0036-8075. PMID 163485.
- ^ a b c d e f g Whitehead, H.; Reeves, R. R.; Tyack, P. L. (2000). "Science and the conversation, protection, and management of wild cetaceans". In Mann, J.; Connor, R. C. (eds.). Cetacean societies : field studies of dolphins and whales. Chicago: University of Chicago Press. ISBN 978-0-226-50340-0. OCLC 42309843.
- ^ Klinowska, Margaret; Cooke, Justin (1991). Dolphins, Porpoises, and Whales of the World: the IUCN Red Data Book (PDF). Columbia University Press, NY: IUCN Publications. ISBN 978-2-88032-936-5. OCLC 24110680.
- ^ a b Thewissen, J. G. M.; Perrin, William R.; Wursig, Bernd (2002). "Hearing". Encyclopedia of Marine Mammals. San Diego: Academic Press. pp. 570–572. ISBN 978-0-12-551340-1.
- ^ U.S. Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service. "Coastal Stock(s) of Atlantic Bottlenose Dolphin: Status Review and Management Proceedings and Recommendations from a Workshop held in Beaufort, North Carolina, 13 September 1993 – 14 September 1993" (PDF). pp. 56–57.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Gregory K. Silber, Dagmar Fertl (1995) – Intentional beaching by bottlenose dolphins (Tursiops truncatus) in the Colorado River Delta, Mexico.
- ^ Marine Mammals: Evolutionary Biology. Academic Press. 2015. p. 430. ISBN 978-0123970022.
{{cite book}}: Cite uses deprecated parameter|authors=(help) - ^ Vogle, A. W.; Lillie, Margo A.; Piscitelli, Marina A.; Goldbogen, Jeremy A.; Pyenson, Nicholas D.; Shadwick, Robert E. (2015). "Stretchy nerves are an essential component of the extreme feeding mechanism of rorqual whales". Current Biology. 25 (9): 360–361. doi:10.1016/j.cub.2015.03.007. PMID 25942546.
- ^ Goldbogen, Jeremy A. (2010). "The Ultimate Mouthful: Lunge Feeding in Rorqual Whales". American Scientist. 98 (2): 124. doi:10.1511/2010.83.124 (inactive 2016-06-05).
{{cite journal}}: CS1 maint: DOI inactive as of June 2016 (link) - ^ Goldbogen, J. A.; Calambokidis, J.; Oleson, E.; Potvin, J.; Pyenson, N. D.; Schorr, G.; Shadwick, R. E. (2011). "Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density". Journal of Experimental Biology. 214 (Pt 1): 131–146. doi:10.1242/jeb.048157. PMID 21147977.
- ^ Perrin 2009, pp. 806–813.
- ^ a b Reitherman, Bruce (Producer and photographer) (1993). Waddlers and Paddlers: A Sea Otter Story–Warm Hearts & Cold Water (Documentary). U.S.A.: PBS.
- ^ Nickerson, p. 21
- ^ Haley, D. (ed.) (1986). "Sea Otter". Marine Mammals of Eastern North Pacific and Arctic Waters (2nd ed.). Seattle, Washington: Pacific Search Press. ISBN 0-931397-14-6. OCLC 13760343.
{{cite book}}:|author=has generic name (help) - ^ "Sea otter". BBC. Retrieved 2007-12-31.
- ^ VanBlaricom, Glenn R. (2001). Sea Otters. Stillwater, MN: Voyageur Press Inc. p. 22. ISBN 978-0-89658-562-1. OCLC 46393741.
- ^ Lavinge, D. M.; Kovacs, K. M.; Bonner, W. N. (2001). "Seals and Sea lions". In MacDonald, D (ed.). The Encyclopedia of Mammals (2nd ed.). Oxford University Press. pp. 147–55. ISBN 978-0-7607-1969-5. OCLC 48048972.
- ^ "Arctic Bears". PBS Nature. 17 February 2008. Archived from the original on 16 June 2008.
{{cite episode}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help); Unknown parameter|serieslink=ignored (|series-link=suggested) (help) - ^ Amstrup, Steven C.; Marcot, Bruce G.; Douglas, David C. (2007). Forecasting the range-wide status of polar bears at selected times in the 21st Century (PDF). Reston, Virginia: U.S. Geological Survey.
- ^ a b Hemstock, Annie (1999). The Polar Bear. Manakato, MN: Capstone Press. pp. 24–27. ISBN 978-0-7368-0031-0. OCLC 38862448.
- ^ Matthews, Downs (1993). Polar Bear. San Francisco: Chronicle Books. ISBN 978-0-8118-0204-8.
- ^ Dyck, M. G.; Romberg, S. (2007). "Observations of a wild polar bear (Ursus maritimus) successfully fishing Arctic charr (Salvelinus alpinus) and Fourhorn sculpin (Myoxocephalus quadricornis)". Polar Biology. 30 (12): 1625–1628. doi:10.1007/s00300-007-0338-3.
- ^ Template:IUCN2008
- ^ Regidor, Héctor A.; Gorostiague, Martín; Sühring, Silvia. "Reproduction and dental age classes of the little water opossum (Lutreolina crassicaudata) in Buenos Aires, Argentina". Revista de Biología Tropical. 47 (1–2): 271–272. ISSN 0034-7744.
- ^ "Water voles get a taste for frogs". BBC News. 30 April 2010. Retrieved 13 June 2016.
- ^ Marsh, Helene; O'Shea, Thomas J.; Reynolds III, John E. (2012). Ecology and Conservation of the Sirenia: Dugongs and Manatees. Cambridge: Cambridge University Press. p. 112. ISBN 978-0-521-88828-8. OCLC 773872519.
- ^ Marsh, Helene. "Dugongidae". Fauna of Australia. Vol. 1. Canberra: Australian Government Public Service. ISBN 978-0-644-06056-1. OCLC 27492815.
- ^ Ringelman, James K. "Managing Beaver to Benefit Waterfowl" (PDF). USGS.gov. United States Geological Survey. Retrieved 2 April 2015.
- ^ Müller-Schwarze, Dietland; Sun, Lixing (2003). The Beaver: Natural History of a Wetlands Engineer. Cornell University Press. pp. 67–75. ISBN 978-0-8014-4098-4.
{{cite book}}: Unknown parameter|lastauthoramp=ignored (|name-list-style=suggested) (help) - ^ Belovsky, Gary E. (1984). "Summer Diet Optimization by Beaver". The American Midland Naturalist. 111 (2): 209–222. doi:10.2307/2425316. ISSN 0003-0031.
- ^ Estes, R. (1992). The Behavior Guide to African Mammals: including hoofed mammals, carnivores, primates. San Francisco: University of California Press. pp. 222–226. ISBN 978-0-520-08085-0. OCLC 19554262.
- ^ "Hippopotamus". Kruger National Park. Retrieved 2007-06-18.
- ^ Grey, J.; Harper, D. M. (2002). "Using Stable Isotope Analyses To Identify Allochthonous Inputs to Lake Naivasha Mediated Via the Hippopotamus Gut". Isotopes in Environmental Health Studies. 38 (4): 245–250. doi:10.1080/10256010208033269. PMID 12725427.
- ^ Eltringham, S. Keith (1999). The Hippos. London: Academic Press. ISBN 978-0-85661-131-5. OCLC 42274422.
- ^ Zschokke, Samuel (2002). "Distorted Sex Ratio at Birth in the Captive Pygmy Hippopotamus, Hexaprotodon Liberiensis". Journal of Mammalogy. 83 (3): 674–681. doi:10.1644/1545-1542(2002)083<0674:DSRABI>2.0.CO;2.
- ^ Laurie, W. A.; Lang, E. M.; Groves, C. P. (1983). "Rhinoceros unicornis" (PDF). Mammalian Species (211). American Society of Mammalogists: 1–6. doi:10.2307/3504002. JSTOR 3504002.
- ^ Howard, Janet L. (1996). "Populus tremuloides". U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory. Retrieved 14 June 2016.
- ^ Saskatchewan Environment and Resource Manager; Canadian Plains Research Center (2001). Natural Neighbours: Selected Mammals of Saskatchewan. Regina, Saskatchewan: University of Regina. p. 170. ISBN 978-0-88977-123-9. ISSN 1498-1297. OCLC 607394660.
- ^ Dalrymple, Byron (1983). North American big-game animals (1 ed.). Stoeger Publishing. p. 84. ISBN 978-08769-1142-6. OCLC 1054473.
- ^ Farb, Peter (1966). The Land and Wildlife of North America. California State department of Education. p. 177. ISBN 978-0809439195.
- ^ Geist, Valerius (1998). Deer of the World: Their Evolution, Behaviour, and Ecology (1 ed.). Machaniesburg, PA: Stackpole Books. p. 237. ISBN 978-0-8117-0496-0. OCLC 37713037.
- ^ a b c d e f Clapham, P. J.; Young, S. B.; Brownell, R. L. (1999). "Baleen whales: Conservation issues and the status of the most endangered populations". Mammal Review. 29: 37–62. doi:10.1046/j.1365-2907.1999.00035.x.
- ^ "History of Whaling". The Húsavík Whale Museum. Retrieved May 16, 2010.
- ^ "Modern Whaling". The Húsavík Whale Museum. Retrieved May 16, 2010.
- ^ Baker, C. S.; Cipriano, F.; Palumbi, S. R. (1996). "Molecular genetic identification of whale and dolphin products from commercial markets in Korea and Japan". Molecular Ecology. 5 (5): 671–685. doi:10.1111/j.1365-294X.1996.tb00362.x.
- ^ a b Riedman, M. (1990). The Pinnipeds: Seals, Sea Lions, and Walruses. San Francisco: University of California Press. ISBN 978-0-520-06497-3. OCLC 19511610.
- ^ Perrin 2009, pp. 585–588.
- ^ Beckman D. W. (2012). Marine Environmental Biology and Conservation. Jones & Bartlett Publishers. p. 315. ISBN 978-0-7637-7350-2. OCLC 613421445.
- ^ Johnson, W. M.; Karamanlidis, A. A.; Dendrinos, P.; de Larrinoa, P. F.; Gazo, M.; González, L. M.; Güçlüsoy, H.; Pires, R.; Schnellmann, M. "Monk Seal Fact Files". monachus-guardian.org. Retrieved September 9, 2013.
- ^ Perrin, W. F. (1994) "Status of species" in Randall R. Reeves and Stephen Leatherwood (eds.) Dolphins, porpoises, and whales: 1994–1998 action plan for the conservation. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources
- ^ Hall, M. A. (1998). "An ecological view of the tuna—dolphin problem: impacts and trade-offs" (PDF). Reviews in Fish Biology and Fisheries. 8: 1–34. doi:10.1023/A:1008854816580.
- ^ a b Anderson, Paul K. (2001). "Marine Mammals in the Next One Hundred Years: Twilight for a Pleistocene Megafauna?". Journal of Mammalogy. 82 (3): 623–629. doi:10.1093/jmammal/82.3.623. JSTOR 1383601.
- ^ a b c d e Wursig, Bernd; Gailey, Glenn A. (2002). "Marine Mammals and Aquaculture: Conflicts and Potential Resolutions". In Stickney, Robert R.; McVey, James P. (eds.). Responsible marine aquaculture. Wallingford, Oxon; New York: CABI. ISBN 978-0-85199-604-2. OCLC 228169018.
- ^ Conn, P. B.; Silber, G. K. (2013). "Vessel speed restrictions reduce risk of collision-related mortality for North Atlantic right whales". Ecosphere. 4 (1). doi:10.1890/ES13-00004.1.
- ^ Constantine, R.; Brunton, D. H.; Dennis, T. (2004). "Dolphin-watching tour boats change bottlenose dolphin (Tursiops truncatus) behaviour". Biological Conservation. 117 (3): 299–307. doi:10.1016/j.biocon.2003.12.009.
- ^ Rosen, D. A.; Trites, A. W. (2000). "Pollock and the decline of Steller sea lions: Testing the junk-food hypothesis". Canadian Journal of Zoology. 78 (7): 1243–1250. doi:10.1139/z00-060.
- ^ McAlpine, D. F.; Stevick, P. T.; Murison, L. D. (1999). "Increase in Extralimital Occurrences of Ice-Breeding Seals in the Northern Gulf of Maine Region: More Seals or Fewer Fish?". Marine Mammal Science. 15 (3): 906–911. doi:10.1111/j.1748-7692.1999.tb00857.x.
- ^ Hutchins, J. (1996). "Spatial and temporal variation in the density of northern cod and a review of hypoth-eses for the stock's collapse" (PDF). Canadian Journal of Fisheries and Aquatic Science. 53 (5): 943–962. doi:10.1139/cjfas-53-5-943.
- ^ Baker, J. R.; Jones, A. M.; Jones, T. P.; Watson, H. C. (1981). "Otter Lutra lutra L. Mortality and marine oil pollution". Biological Conservation. 20 (4): 311–321. doi:10.1016/0006-3207(81)90017-3.
- ^ Harwood, J. (2001). "Marine Mammals and their Environment in the Twenty-First Century". Journal of Mammalogy. 82 (3): 630–640. doi:10.1644/1545-1542(2001)082<0630:MMATEI>2.0.CO;2. JSTOR 1383602.
- ^ Madronich, S.; McKenzie, R. L.; Björn, L. O.; Caldwell, M. M. (1998). "Changes in biologically active ultraviolet radiation reaching the Earth's surface". Journal of Photochemistry and Photobiology B: Biology. 46: 5–19. doi:10.1016/S1011-1344(98)00182-1.
- ^ Simmonds, M. P.; Isaac, S. J. (2007). "The impacts of climate change on marine mammals: Early signs of significant problems". Oryx. 41: 19. doi:10.1017/S0030605307001524.
- ^ Stirling, Ian; Lunn, N. J.; Iacozza, J. (September 1999). "Long-term trends in the population ecology of polar bears in Western Hudson Bay in relation to climatic change" (PDF). Arctic. 52 (3): 294–306. doi:10.14430/arctic935.
- ^ Amstrup, S. C.; Marcot, B. G.; Douglas, D. C. (2008). DeWeaver, Eric L.; Bitz, Cecilia M.; Tremblay, L.-Bruno (eds.). "A Bayesian Network Modeling Approach to Forecasting the 21st Century Worldwide Status of Polar Bears" (PDF). Arctic Sea Ice Decline: Observations, Projections, Mechanisms, and Implications. American Geophysical Union. doi:10.1029/180GM14.
{{cite journal}}: Cite journal requires|journal=(help)
Further reading
- Perrin, W. F.; Wursig, B.; Thewissen, J. G. M. (2009). Encyclopedia of Marine Mammals. Academic Press. ISBN 978-0-0809-1993-5.
External links
- The Society for Marine Mammalogy The largest organization of marine mammalogists in the world.
- Introduction to the Desmostylia Museum of Paleontology, University of California – extinct group of marine mammals








