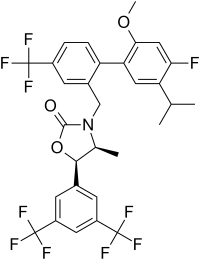
Anacetrapib
Appearance
| |
| Names | |
|---|---|
| Preferred IUPAC name
(4S,5R)-5-[3,5-Bis(trifluoromethyl)phenyl]-3-{[4′-fluoro-2′-methoxy-5′-(propan-2-yl)-4-(trifluoromethyl)[1,1′-biphenyl]-2-yl]methyl}-4-methyl-1,3-oxazolidin-2-one | |
| Other names
MK-0859
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H25F10NO3 | |
| Molar mass | 637.51 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anacetrapib is a CETP inhibitor which was being developed to treat elevated cholesterol levels in an effort to prevent cardiovascular disease.[1] In 2017 its development was abandoned by Merck.[2]
Evidence
In 2017 REVEAL trial anacetrapib was shown to decrease the risk of repeat heart attacks in high-risk patients with previous acute coronary events.[3]
See also
Other CETP inhibitors:
- Torcetrapib was developed by Pfizer until December 2006 but caused unacceptable increases in blood pressure and had net cardiovascular detriment.
- Dalcetrapib was developed by Hoffmann–La Roche until May 2012. It did not raise blood pressure and did raise HDL, but it showed no clinically meaningful efficacy.
- Evacetrapib was developed by Eli Lilly & Company until October 2015.
References
- ^ Gutstein DE, Krishna R, Johns D, et al. (2012). "Anacetrapib, a Novel CETP Inhibitor: Pursuing a New Approach to Cardiovascular Risk Reduction". Clinical Pharmacology & Therapeutics. 91 (1): 109–122. doi:10.1038/clpt.2011.271. PMID 22130116. S2CID 36510986.
- ^ "Merck says will not seek approval of cholesterol treatment". Reuters. 2017. Retrieved 18 October 2017.
- ^ Filippatos, TD; Kei, A; Elisaf, MS (29 September 2017). "Anacetrapib, a New CETP Inhibitor: The New Tool for the Management of Dyslipidemias?". Diseases. 5 (4): 21. doi:10.3390/diseases5040021. PMC 5750532. PMID 28961179.
Further reading
- WO 2007005572, Miller, Ross A. & Cote, Aaron S., "Process for synthesizing a CETP inhibitor", published 2007-01-11, assigned to Merck & Co. Inc.
