IQGAP1
Ras GTPase-activating-like protein IQGAP1 (IQGAP1) also known as p195 is a ubiquitously expressed protein that in humans is encoded by the IQGAP1 gene.[5][6][7] IQGAP1 is a scaffold protein involved in regulating various cellular processes ranging from organization of the actin cytoskeleton, transcription, and cellular adhesion to regulating the cell cycle.
History
IQGAP1 was discovered in 1994.[5] Its name stems from the fact that its RasGAP-related domain (GRD) has sequence homology to the Sar1 GTPase.[8] It was hypothesized that IQGAP1 would act as a GTPase activating protein (GAP) protein, promoting the switch of ras GTPases from the active GTP to GDP-bound forms. However, despite the homology of IQGAP’s GAP domain to sar1 and the fact that IQGAP1 binds Rho GTPases Rac1 and Cdc42, IQGAP does not actually have GAP function. Instead, it binds the active (GTP-bound) forms of RAC1 and CDC42 with higher affinity than GDP-bound forms, and stabilizes the active form in vivo.[9]
IQGAP1 is now recognized as a protein scaffold that integrates signals regulating cell adhesion, actin cytoskeleton, the cell cycle,[9] and other cellular functions. IQGAP is particularly interesting as a therapeutic target since it acts as a node for so many signaling pathways implicated in cancer progression.
Expression
Analysis of IQGAP1 expression in human tissues has indicated that the scaffold is more or less ubiquitously expressed.[10] It is usually found in the nucleus, plasma membrane, and cytoplasm. In other words it is found throughout the cell as well as throughout tissue types. Expression analysis has also indicated that IQGAP1 is overexpressed in many cancers, and in more aggressive colorectal and ovarian cancers, IQGAP1 is localized at the invasive front of the neoplasm, indicating a role in mobilization of the cells.[8] Importantly, approximately 10% of genes that show increased expression in metastatic cells are IQGAP1 binding partners.[8]
Domains
IQGAP1 is a 190 kDa protein with 5 domains.[9] A protein domain is a subsection of a protein that shows up multiple times in biology and can exist independently of the surrounding protein. It is very similar to subsections of other proteins, and could be cut out of the current protein, exist and function by itself, or be pasted in to a new protein strand and still function properly. Since this area of the protein is conserved in amino acid sequence and structure, it can be characterized by function or binding partner. IQGAP1 has 5 well-known domains separated by other amino acids.

Starting at the N-terminus (or front of the protein), IQGAP1 contains a calponin homology domain (CHD), which mediates actin-binding[11] and binds calponin.
The WW, or poly-proline protein-protein domain, so named because of two functionally conserved tryptophans, W, is a protein-protein interaction domain that associates with proline-rich regions of other proteins.[12][13]
The WW domain is followed by 4 IQ motifs which form an IQ domain. This domain binds calmodulin,[14] a protein known as a calcium sensor that can bind and regulate many target proteins.[15]
A GRD (rasGAP-related domain) follows the IQ domain. This domain is highly similar to the functional subunit of Ras GTPase-activating proteins (GAPs) and was thus thought to have GAP function. IQGAP1 does bind Rho GTPases CDC42 and RAC1, however, IQGAP1 is unusual in that it actually has no GAP function, and instead stabilizes the GTP-bound proteins in their active state.[16]
Finally, IQGAP1 has a RasGAP_c carboxy terminal sequence important for binding Beta-catenin and E-cadherin.[9]
Related Proteins
Homologues of IQGAP1 are known in species as divergent as yeast, worms, and humans (as well as other mammals), though the domains are not always highly conserved.[9]
IQGAP1 is the most well studied member of the IQGAP family of scaffold proteins. The two other members of the family include IQGAP2 and IQGAP3 which have far more restricted expression patterns in comparison with IQGAP1. IQGAP2 is found in the liver, stomach, and platelets and is 62% identical to IQGAP1,[9] but appears to have a drastically divergent function in terms of pathology.[17]
In the brain, IQGAP3 appears to play an important role in neuronal morphogenesis.[18]
Function
This gene encodes a member of the IQGAP family. The protein contains four IQ domains, one calponin homology domain, one Ras-GAP domain and one WW domain. It interacts with components of the cytoskeleton[19] such as the formin Dia1 (mDia1),[20] with cell adhesion molecules (CAMs), and with several signaling molecules to regulate cell morphology and motility. For example, IQGAP1 expression is necessary for neuronal process outgrowth on the cell adhesion molecule PTPmu (PTPRM).[21] Expression of the protein is upregulated by gene amplification in two gastric cancer cell lines[7] and its over-expression and distinct membrane localisation is also observed in a range of tumours.[22]
Interactions
IQGAP1 is a node intersected by many signaling pathways. As such it has many binding partners, many of which have essential roles in control of the cell cycle and actin cytoskeleton.
IQGAP1 has been shown to interact with:
- Calmodulin 1,[23][24]
- CDC42,[6][25][26][27][28]
- CDH1,[29]
- CLIP1,[26]
- PRKACA,[30]
- RAC1,[6][25][26][28] and
- S100B.[31]
- Actin – cytoskeletal structure
- ARF6
- APC
- Beta-catenin–cell adhesion and WNT signaling: transcription
- B-raf – MAPK pathway
- CD44
- Erk1/2 – MAPK pathway, cell cycle control, proliferation
- Mek ½ -- MAPK pathway, cell cycle control, proliferation
- Src
- PTPmu (PTPRM)[21]
- complete list at [32]
Function as a Scaffold
Protein binding does not by itself construct an interesting story. Far more important is the outcome of the binding event. Does binding change the target protein’s localization? Does it activate the target, or in some way change the target (or effector molecule’s) conformation? As a scaffolding protein, IQGAP1 binds and regulates many targets—its role is to integrate and mediate signaling from diverse pathways and insulate key pathway members from crosstalk.
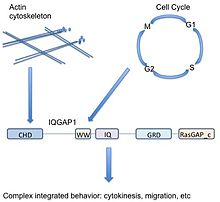
Scaffolds organize signaling pathways—help regulate how various extracellular signals can be transduced by the same canonical pathway members into various cellular outputs.[33] Generally, scaffolds regulate output, localization, and selectivity of pathways.[34]
As a scaffold involved in different signaling pathways (actin cytoskeleton, cellular adhesion, cell cycle, transcription), IQGAP1 has a unique ability to potentially couple diverse cellular functions. For example IQGAP1 is associated with actin dynamics through direct binding of actin and indirect regulation via Cdc42/Rac1, but also modulates the MAPK pathway which is associated with cell cycle control. Thus IQGAP1 may couple MAPK signaling (decisions about cell fate) to the cytoskeleton or cellular adhesion (potentially acting out those decisions)—an important implication for cancer.
To simplify, due to its diverse range of binding partners, IQGAP1 may act as a link between logically related but molecularly distinct cellular functions. In the above example, actin cytoskeleton rearrangement is required for proliferation (cytokinesis during mitosis). IQGAP1 helps cells both listen to and act on signals, playing an integral role in connecting the dots between signals for proliferation and the actual cellular response.
Key pathways
ERK MAPK
The Ras→Raf→MEK→ERK MAPK signaling pathway plays an integral part in the processes of cell proliferation, differentiation, and apoptosis. This pathway is conserved across all eukaryotes.
Various extracellular signals induce the ERK MAPK pathway including EGF, IGF-1, PDGF, and NGF.[33] The various scaffolds of this pathway, including IQGAP1, are responsible for modulating the cellular response to the activity of this pathway. For instance, in a given cell line, activation by one extracellular signal may induce differentiation but not proliferation, while activation of the same ERK MAPK pathway by a different extracellular signal will induce proliferation but not differentiation.[33] IQGAP1 seems to be responsible for the specific output of the pathway upon activation by EGF.
IQGAP1 plays a significant role in the propagation of this MAPK signaling pathway. IQGAP directly binds b-RAF,[35] MEK1/2 and ERK1/2, and is in fact necessary for the phosphorylation (activation) of ERK upon stimulation by EGF.[36][37]
Cytoskeletal control (actin dynamics)
Actin is a major building block of every eukaryotic cell’s cytoskeleton. Actin dynamics play a major role in cell motility (filaments are built at the leading edge of a moving cell and deconstructed at the receding edge). IQGAP1 binds actin and influences actin dynamics by localizing to the leading edge and recruiting actin polymerization machinery.[8][9][19]
IQGAP1 binds and is a target of the Rho GTPases CDC42 and RAC1 which are well known regulators of the actin cytoskeleton.[38][39] Despite its name, IQGAP1 does not have GAP function, and instead stabilizes active Cdc42. This increase in a local pool of active Cdc42 stimulates actin filament formation and thus filopodia formation.[9]
IQGAP1 can crosslink actin,[40] and in many organisms, IQGAP1 is involved in cytokinesis.[41]
Adhesion
Cadherins are a family of adhesion proteins that localize to the cell surface where they anchor a cell to its neighbors by clasping on to the extracellular portion of the neighbor’s cadherins. Actin binds a-catenin which binds beta-catenin which in turn binds E-cadherin. E-cadherin juts into the extracellular space to grasp the extracellular domains of neighboring E-cadherins. IQGAP1 localizes to cell-cell contacts and binds actin, b-catenin, and E-cadherin, weakening these junctions and thus decreasing cell-cell adhesion.[9][42] IQGAP weakens cell adhesion by displacing a-catenin from the complex.[43]
Active RAC1 binds IQGAP1 to crosslink actin filaments and prevents IQGAP1 from interacting with beta-catenin, stabilizing cell-cell contacts.[44] When IQGAP1 does not bind Rac1, however, it binds beta-catenin, displacing a-catenin from the cadherin-catenin cellular adhesion complex.
Transcription
IQGAP1 also affects transcription through the Wnt signaling pathway by its interaction with beta-catenin.[8] Beta-catenin is usually sequestered in a complex and excluded from the nucleus, but upon WNT activation this complex is broken and beta-catenin translocates to the nucleus where it activates transcriptional programs. IQGAP1 binds b-catenin and increases nuclear localization and expression of beta-catenin’s transcriptional targets.
Clinical significance
IQGAP1 is associated with cytoskeletal dynamics, transcription, cell adhesion, cell cycle, and morphology, all of which are disrupted in cancer. As a modulatory protein intersecting all of these pathways, IQGAP1 can couple many of them, and is also responsible for their proper propagation. Since cancer is a disease characterized by the perturbation of many of these cellular processes, IQGAP1 is a logical oncogene candidate and therapeutic target.
Expression analysis has implicated IQGAP1 in colorectal, squamous cell, breast, gastric, liver, lung, and ovarian cancers,[45] and in some of these cancers, higher IQGAP1 expression levels indicate a poor prognosis.[46]
In order for a cancer to metastasize, cells must gain migratory abilities and invade other tissues. Through Rac1/CDC42, IQGAP1 regulates cellular adhesion and actin dynamics.
In normal cells IQGAP1 localizes to areas of high actin turnover. This characteristic is echoed in invasive tissues, where IQGAP1 localizes to the leading edge of migrating cells.[8] Over-expression of IQGAP1 was associated with increased migration and invasion in a human breast epithelial cancer cell line (MCF-7 cells).[8][47] IQGAP1 may also be involved in the deregulation of proliferation and differentiation through its modulation of the ERK MAPK pathway.
IQGAP1 may be necessary for tumorigenesis. IQGAP1 knockdown in MCF-7 cancer cells reduced the malignant phenotype (serum-dependent proliferation and anchorage independent growth). 100% of mice injected with MCF-7 cells overexpressing IQGAP1 developed tumors and these tumors were highly invasive. Control MCF-7 cells formed tumors in 60% of the mice, and MCF-7 cells with stable knockdown of IQGAP1 only formed tumors 20% of the time.[47] The mechanism for how IQGAP1 may modulate tumorigenesis/invasion through its various binding partners is of great interest.
IQGAP1 null mice appear significantly normal, with the only life history abnormality being an increase in gastric hyperplasia.[48] Thus, IQGAP1 may be an effective therapeutic target, if its knockdown has little effect in homeostatic tissue but its expression is important in cancer.
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000140575 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000030536 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A (Sep 1994). "Identification of a human rasGAP-related protein containing calmodulin-binding motifs". J. Biol. Chem. 269 (32): 20517–21. doi:10.1016/S0021-9258(17)32023-9. PMID 8051149.
- ^ a b c Hart MJ, Callow MG, Souza B, Polakis P (Aug 1996). "IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs". EMBO J. 15 (12): 2997–3005. doi:10.1002/j.1460-2075.1996.tb00663.x. PMC 450241. PMID 8670801.
- ^ a b "Entrez Gene: IQGAP1 IQ motif containing GTPase activating protein 1".
- ^ a b c d e f g White CD, Brown MD, Sacks DB (June 2009). "IQGAPs in cancer: a family of scaffold proteins underlying tumorigenesis". FEBS Lett. 583 (12): 1817–24. doi:10.1016/j.febslet.2009.05.007. PMC 2743239. PMID 19433088.
- ^ a b c d e f g h i Briggs MW, Sacks DB (June 2003). "IQGAP proteins are integral components of cytoskeletal regulation". EMBO Rep. 4 (6): 571–4. doi:10.1038/sj.embor.embor867. PMC 1319206. PMID 12776176.
- ^ "IQGAP1: Gene and protein summary". The Human Protein Atlas. Retrieved 2011-05-31.
- ^ Stradal T, Kranewitter W, Winder SJ, Gimona M (July 1998). "CH domains revisited". FEBS Lett. 431 (2): 134–7. doi:10.1016/S0014-5793(98)00751-0. PMID 9708889. S2CID 29513846.
- ^ Sudol M, Chen HI, Bougeret C, Einbond A, Bork P (August 1995). "Characterization of a novel protein-binding module--the WW domain". FEBS Lett. 369 (1): 67–71. doi:10.1016/0014-5793(95)00550-S. PMID 7641887.
- ^ Macias MJ, Wiesner S, Sudol M (February 2002). "WW and SH3 domains, two different scaffolds to recognize proline-rich ligands". FEBS Lett. 513 (1): 30–7. doi:10.1016/S0014-5793(01)03290-2. PMID 11911877.
- ^ Rhoads AR, Friedberg F (April 1997). "Sequence motifs for calmodulin recognition". FASEB J. 11 (5): 331–40. doi:10.1096/fasebj.11.5.9141499. PMID 9141499. S2CID 1877645.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stevens FC (August 1983). "Calmodulin: an introduction". Can. J. Biochem. Cell Biol. 61 (8): 906–10. doi:10.1139/o83-115. PMID 6313166.
- ^ Kurella VB, Richard JM, Parke CL, Lecour LF, Bellamy HD, Worthylake DK (May 2009). "Crystal structure of the GTPase-activating protein-related domain from IQGAP1". J. Biol. Chem. 284 (22): 14857–65. doi:10.1074/jbc.M808974200. PMC 2685667. PMID 19321438.
- ^ White CD, Khurana H, Gnatenko DV, Li Z, Odze RD, Sacks DB, Schmidt VA (2010). "IQGAP1 and IQGAP2 are reciprocally altered in hepatocellular carcinoma". BMC Gastroenterol. 10: 125. doi:10.1186/1471-230X-10-125. PMC 2988069. PMID 20977743.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Wang S, Watanabe T, Noritake J, Fukata M, Yoshimura T, Itoh N, Harada T, Nakagawa M, Matsuura Y, Arimura N, Kaibuchi K (February 2007). "IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth". J. Cell Sci. 120 (Pt 4): 567–77. doi:10.1242/jcs.03356. PMID 17244649.
- ^ a b Brandt DT, Grosse R (November 2007). "Get to grips: steering local actin dynamics with IQGAPs". EMBO Rep. 8 (11): 1019–23. doi:10.1038/sj.embor.7401089. PMC 2247391. PMID 17972901.
- ^ Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R (July 2007). "Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation". J. Cell Biol. 178 (2): 193–200. doi:10.1083/jcb.200612071. PMC 2064439. PMID 17620407.
- ^ a b Phillips-Mason PJ, Gates TJ, Major DL, Sacks DB, Brady-Kalnay SM (2006). "The receptor protein-tyrosine phosphatase PTPmu interacts with IQGAP1". The Journal of Biological Chemistry. 281 (8): 4903–10. doi:10.1074/jbc.M506414200. PMID 16380380.
- ^ Johnson M, Sharma M, Henderson BR (March 2009). "IQGAP1 regulation and roles in cancer". Cell. Signal. 21 (10): 1471–8. doi:10.1016/j.cellsig.2009.02.023. PMID 19269319.
- ^ Li Z, Sacks DB (February 2003). "Elucidation of the interaction of calmodulin with the IQ motifs of IQGAP1". J. Biol. Chem. 278 (6): 4347–52. doi:10.1074/jbc.M208579200. PMID 12446675.
- ^ Briggs MW, Li Z, Sacks DB (March 2002). "IQGAP1-mediated stimulation of transcriptional co-activation by beta-catenin is modulated by calmodulin". J. Biol. Chem. 277 (9): 7453–65. doi:10.1074/jbc.M104315200. PMID 11734550.
- ^ a b Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K (September 1996). "Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1". J. Biol. Chem. 271 (38): 23363–7. doi:10.1074/jbc.271.38.23363. PMID 8798539.
- ^ a b c Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K (June 2002). "Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170". Cell. 109 (7): 873–85. doi:10.1016/S0092-8674(02)00800-0. PMID 12110184.
- ^ Joyal JL, Annan RS, Ho YD, Huddleston ME, Carr SA, Hart MJ, Sacks DB (June 1997). "Calmodulin modulates the interaction between IQGAP1 and Cdc42. Identification of IQGAP1 by nanoelectrospray tandem mass spectrometry". J. Biol. Chem. 272 (24): 15419–25. doi:10.1074/jbc.272.24.15419. PMID 9182573.
- ^ a b Zhang B, Chernoff J, Zheng Y (April 1998). "Interaction of Rac1 with GTPase-activating proteins and putative effectors. A comparison with Cdc42 and RhoA". J. Biol. Chem. 273 (15): 8776–82. doi:10.1074/jbc.273.15.8776. PMID 9535855.
- ^ Li Z, Kim SH, Higgins JM, Brenner MB, Sacks DB (December 1999). "IQGAP1 and calmodulin modulate E-cadherin function". J. Biol. Chem. 274 (53): 37885–92. doi:10.1074/jbc.274.53.37885. PMID 10608854.
- ^ Nauert JB, Rigas JD, Lester LB (September 2003). "Identification of an IQGAP1/AKAP79 complex in beta-cells". J. Cell. Biochem. 90 (1): 97–108. doi:10.1002/jcb.10604. PMID 12938160. S2CID 83939808.
- ^ Mbele GO, Deloulme JC, Gentil BJ, Delphin C, Ferro M, Garin J, Takahashi M, Baudier J (December 2002). "The zinc- and calcium-binding S100B interacts and co-localizes with IQGAP1 during dynamic rearrangement of cell membranes". J. Biol. Chem. 277 (51): 49998–50007. doi:10.1074/jbc.M205363200. PMID 12377780.
- ^ "IQGAP1 IQ motif containing GTPase activating protein 1 [Homo sapiens (Human)] - Gene - NCBI".
- ^ a b c Sacks DB (November 2006). "The role of scaffold proteins in MEK/ERK signalling". Biochem. Soc. Trans. 34 (Pt 5): 833–836. doi:10.1042/BST0340833. PMID 17052209.
- ^ Good MC, Zalatan JG, Lim WA (May 2011). "Scaffold proteins: hubs for controlling the flow of cellular information". Science. 332 (6030): 680–6. Bibcode:2011Sci...332..680G. doi:10.1126/science.1198701. PMC 3117218. PMID 21551057.
- ^ Ren JG, Li Z, Sacks DB (June 2007). "IQGAP1 modulates activation of B-Raf". Proc. Natl. Acad. Sci. U.S.A. 104 (25): 10465–9. Bibcode:2007PNAS..10410465R. doi:10.1073/pnas.0611308104. PMC 1965536. PMID 17563371.
- ^ Roy M, Li Z, Sacks DB (April 2004). "IQGAP1 binds ERK2 and modulates its activity". J. Biol. Chem. 279 (17): 17329–37. doi:10.1074/jbc.M308405200. PMID 14970219.
- ^ Roy M, Li Z, Sacks DB (September 2005). "IQGAP1 is a scaffold for mitogen-activated protein kinase signaling". Mol. Cell. Biol. 25 (18): 7940–52. doi:10.1128/MCB.25.18.7940-7952.2005. PMC 1234344. PMID 16135787.
- ^ Hall A (May 1992). "Ras-related GTPases and the cytoskeleton". Mol. Biol. Cell. 3 (5): 475–9. doi:10.1091/mbc.3.5.475. PMC 275601. PMID 1611153.
- ^ Narumiya S (August 1996). "The small GTPase Rho: cellular functions and signal transduction". J. Biochem. 120 (2): 215–28. doi:10.1093/oxfordjournals.jbchem.a021401. PMID 8889802.
- ^ Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K (November 1997). "Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42". J. Biol. Chem. 272 (47): 29579–83. doi:10.1074/jbc.272.47.29579. PMID 9368021.
- ^ Machesky LM (March 1998). "Cytokinesis: IQGAPs find a function". Curr. Biol. 8 (6): R202–5. doi:10.1016/S0960-9822(98)70125-3. PMID 9512410.
- ^ Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K (August 1998). "Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion". Science. 281 (5378): 832–5. Bibcode:1998Sci...281..832K. doi:10.1126/science.281.5378.832. PMID 9694656.
- ^ Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K (September 1999). "Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin". J. Biol. Chem. 274 (37): 26044–50. doi:10.1074/jbc.274.37.26044. PMID 10473551.
- ^ Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K (May 2005). "IQGAP1: a key regulator of adhesion and migration". J. Cell Sci. 118 (Pt 10): 2085–92. doi:10.1242/jcs.02379. PMID 15890984.
- ^ Human protein atlas: http://www.proteinatlas.org/ENSG00000140575
- ^ McDonald KL, O'Sullivan MG, Parkinson JF, Shaw JM, Payne CA, Brewer JM, Young L, Reader DJ, Wheeler HT, Cook RJ, Biggs MT, Little NS, Teo C, Stone G, Robinson BG (May 2007). "IQGAP1 and IGFBP2: valuable biomarkers for determining prognosis in glioma patients". J. Neuropathol. Exp. Neurol. 66 (5): 405–17. doi:10.1097/nen.0b013e31804567d7. PMID 17483698.
- ^ a b Jadeski L, Mataraza JM, Jeong HW, Li Z, Sacks DB (January 2008). "IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells". J. Biol. Chem. 283 (2): 1008–17. doi:10.1074/jbc.M708466200. PMID 17981797.
- ^ Li S, Wang Q, Chakladar A, Bronson RT, Bernards A (January 2000). "Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1". Mol. Cell. Biol. 20 (2): 697–701. doi:10.1128/mcb.20.2.697-701.2000. PMC 85173. PMID 10611248.
Further reading
- Tirnauer JS (2004). "A new cytoskeletal connection for APC: linked to actin through IQGAP". Dev. Cell. 7 (6): 778–80. doi:10.1016/j.devcel.2004.11.012. PMID 15572120.
- McCallum SJ, Wu WJ, Cerione RA (1996). "Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 and a Cdc42Hs binding partner with similarity to IQGAP2". J. Biol. Chem. 271 (36): 21732–7. doi:10.1074/jbc.271.36.21732. PMID 8702968.
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS (1997). "IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments". J. Cell Biol. 137 (7): 1555–66. doi:10.1083/jcb.137.7.1555. PMC 2137827. PMID 9199170.
- McCallum SJ, Erickson JW, Cerione RA (1998). "Characterization of the association of the actin-binding protein, IQGAP, and activated Cdc42 with Golgi membranes". J. Biol. Chem. 273 (35): 22537–44. doi:10.1074/jbc.273.35.22537. PMID 9712880.
- Sugimoto N, Imoto I, Fukuda Y, Kurihara N, Kuroda S, Tanigami A, Kaibuchi K, Kamiyama R, Inazawa J (2001). "IQGAP1, a negative regulator of cell-cell adhesion, is upregulated by gene amplification at 15q26 in gastric cancer cell lines HSC39 and 40A". J. Hum. Genet. 46 (1): 21–5. doi:10.1007/s100380170119. PMID 11289714.
- Nabeshima K, Shimao Y, Inoue T, Koono M (2002). "Immunohistochemical analysis of IQGAP1 expression in human colorectal carcinomas: its overexpression in carcinomas and association with invasion fronts". Cancer Lett. 176 (1): 101–9. doi:10.1016/S0304-3835(01)00742-X. PMID 11790459.
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS (2002). "The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin". J. Biol. Chem. 277 (14): 12324–33. doi:10.1074/jbc.M109535200. PMID 11809768.
- Swart-Mataraza JM, Li Z, Sacks DB (2002). "IQGAP1 is a component of Cdc42 signaling to the cytoskeleton". J. Biol. Chem. 277 (27): 24753–63. doi:10.1074/jbc.M111165200. PMID 11948177.
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R (July 2007). "Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation". J. Cell Biol. 178 (2): 193–200. doi:10.1083/jcb.200612071. PMC 2064439. PMID 17620407.









