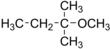
tert-Amyl methyl ether
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methoxy-2-methylbutane | |||
| Other names
tertiary-Amyl methyl ether; TAME; Methoxypentane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | TAME | ||
| ChemSpider | |||
| ECHA InfoCard | 100.012.374 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H14O | |||
| Molar mass | 102.177 g·mol−1 | ||
| Appearance | Clear, colorless liquid | ||
| Density | 0.76-0.78 g/mL[3] | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 86.3 °C (187.3 °F; 359.4 K) | ||
| 10.71 g/L at 20 °C | |||
Refractive index (nD)
|
1.3896 | ||
| Hazards | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 430 °C (806 °F; 703 K) | |||
| Explosive limits | 1.0-7.1% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha.[4] It has an ethereous odor.[1] Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.[5]
Other names:[6]
- 2-Methoxy-2-methylbutane
- Butane, 2-methoxy-2-methyl-
- 1,1-Dimethylpropyl methyl ether
- Methyl tert-pentyl ether
- Methyl tert-Amyl ether
- 2-Methyl-2-methoxybutane
- Methyl 2-methyl-2-butyl ether
- tert-Pentyl methyl ether
- Tertiary amyl methyl ether
- Methyl 1,1-dimethylpropyl ether
- 2-Methoxy-2-methylbutane
Uses[edit]
TAME is mostly used as an oxygenate to gasoline. It is added for three reasons: to increase octane enhancement, to replace banned tetraethyl lead, and to raise the oxygen content in gasoline. It is known that TAME in fuel reduces exhaust emissions of some volatile organic compounds.[1]
TAME is also used as a solvent in organic synthesis as a more environmentally friendly alternative to some of the classic ether solvents.[4] It is characterized by a high boiling point (86°C) and a low freezing point (−80°C), allowing a wide range of reaction temperatures. TAME can be used as a safe reaction medium (e.g. condensation reactions, coupling reactions, such as Grignard reactions and Suzuki reactions, as well as metal hydride reductions) and as an extraction solvent to replace dichloromethane, aromatics, and other ethers.[7][failed verification]
A series of experiments were carried out in a batch reactor at the temperature range of 313-343 K to study the synthesis of tert-amyl ethyl ether from ethanol (EtOH) and 2-methyl-1-butene (2M1B) catalyzed by the NKC-9 ion-exchange resin. The suitable reaction pressure was obtained by using the method of the Gibbs free energy minimization. The activity coefficients of each component were accurately calculated using the Wilson method, then, the equilibrium constants was obtained. The effect of catalyst size, stirring rate, temperature and EtOH/2M1B molar ratio was investigated at the chosen pressure, respectively. A kinetic model which considered the variation of each component volume was established. The method of nonlinear least square combined with genetic algorithm (NLS-GA) was proposed to estimate the kinetic constant in the forward direction. Results indicated that simulated kinetics results were agreed well with the experimental data.
Toxicity[edit]
This section needs expansion. You can help by adding to it. (July 2015) |
TAME was evaluated in 4-week rat inhalation studies sponsored by Amoco Corporation. Target vapor concentrations were 0, 500, 2000, or 4000 ppm for 6 h per day, 5 days per week, for 4 weeks. Exposure at 4000 ppm resulted in 25% mortality, apparently as a consequence of severe CNS depression. Body weight gain was decreased in the TAME high dose male rats. Significant effects on functional observational battery (FOB) parameters were only found in the high and mid-dose groups immediately after exposure. All affected FOB parameters were normal by the next day. TAME exposure significantly increased relative liver weights in the high and mid-dose groups. However, no treatment-related histopathologic findings were noted for the compound. Clinical chemistry and hematology findings were minimal with TAME exposure. The results indicate that 500 ppm was a NOAEL for TAME in these studies.[8]
Some other properties[6][edit]
Relative vapor density (air = 1): 3.6
Vapor Pressure 75.2 [mmHg]
log Kow = 1.55 at 20 °C
Henry's Law constant = 1.32X10-3 atm-cu m/mol at 25 °C
Stability / Shelf Life: Stable under recommended storage conditions.
Autoignition Temperature: 415 °C
Decomposition: When heated to decomposition it emits acrid smoke and irritating vapors.
Odor Threshold: 0.02 [mmHg]
Kovats retention index[edit]
Standard non-polar 672.5, 674, 673, 669.3, 666
Semi-standard non-polar 678, 655, 668.3
Standard polar 790, 802.9
See also[edit]
References[edit]
- ^ a b c "tert-AMYL METHYL ETHER (1,1-DIMETHYLPROPYL METHYL ETHER)". chemicalland21.com. Retrieved 2009-10-20.
- ^ National Industrial Chemicals Notification and Assessment Scheme (2001). "t-Amyl methyl ether (TAME)" (PDF). Full Public Reports. Retrieved 2009-10-20.
- ^ "tert-Amyl methyl ether". Sigma-Aldrich.
- ^ a b Prat, Denis; Wells, Andy; Hayler, John; Sneddon, Helen; McElroy, C. Robert; Abou-Shehada, Sarah; Dunn, Peter J. (2015-12-21). "CHEM21 selection guide of classical- and less classical-solvents". Green Chem. 18 (1): 288–296. doi:10.1039/c5gc01008j. ISSN 1463-9270.
- ^ Diaz, Arthur F.; Drogos, Donna L. (2001-11-06). Oxygenates in Gasoline. ACS Symposium Series. Vol. 799. American Chemical Society. pp. 138–152. doi:10.1021/bk-2002-0799.ch010. ISBN 978-0841237605.
- ^ a b PubChem. "tert-Amyl methyl ether". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-10-22.
- ^ "INEOS Oligomers Products". INEOS. Archived from the original on 2017-11-07. Retrieved 2017-10-30.
- ^ White, Russell D.; Daughtrey, Wayne C.; Wells, Mike S. (December 1995). "Health effects of inhaled tertiary amyl methyl ether and ethyl tertiary butyl ether". Toxicology Letters. 82–83: 719–724. doi:10.1016/0378-4274(95)03590-7. PMID 8597132.