User:Ikevin505/sandbox
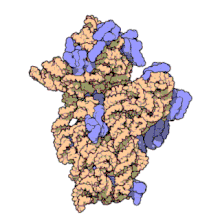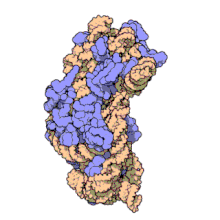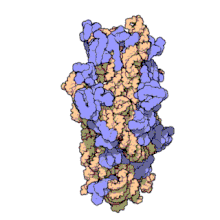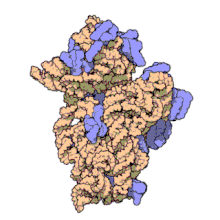
16S ribosomal RNA (or 16S rRNA) is a component of the 30S small subunit of prokaryotic ribosomes. It is 1.542kb (or 1542 nucleotides) in length.[2] The genes coding for it are referred to as 16S rDNA and are used in reconstructing phylogenies, thanks to the work of Carl Woese and George E. Fox.[3]
Multiple sequences of 16S rRNA can exist within a single bacterium.[4]
Functions[edit]
It has several functions:
- Like the large (23S) ribosomal RNA, it has a structural role, acting as a scaffold defining the positions of the ribosomal proteins.
- The 3' end contains the anti-Shine-Dalgarno sequence, which binds upstream to the AUG start codon on the mRNA. The 3'-end of 16S RNA binds to the proteins S1 and S21 known to be involved in initiation of protein synthesis; RNA-protein cross-linking by A.P. Czernilofsky et al. (FEBS Lett. Vol 58, pp 281–284, 1975).
- Interacts with 23S, aiding in the binding of the two ribosomal subunits (50S+30S)
- Stabilizes correct codon-anticodon pairing in the A site, via a hydrogen bond formation between the N1 atom of Adenine (see image of Purine chemical structure) residues 1492 and 1493 and the 2'OH group of the mRNA backbone
Structure[edit]
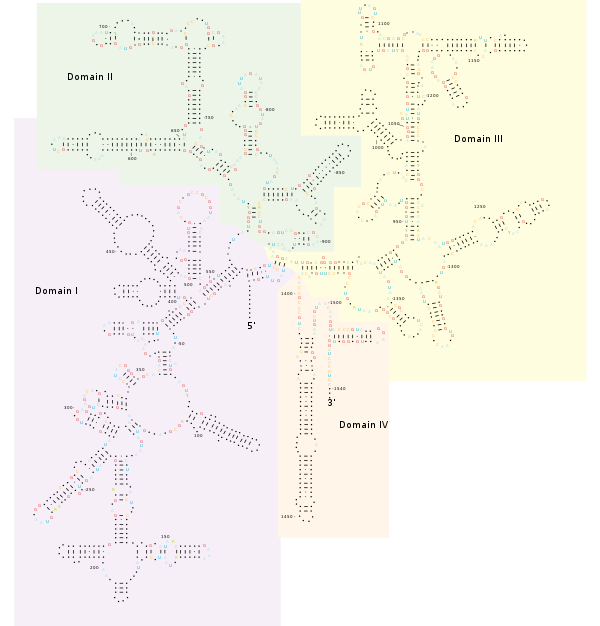

Conformational Change[edit]
A-site Binding[edit]
Specific binding of aminoglycoside antibiotics induces a local conformational change in the A-site of 16S rRNA within the prokaryotic 30S ribosomal subunit; this conformational change causes codon misreading and inhibits translocation [5]. Aminoglycosides are a special class of antibiotics that are used to treat certain bacterial infections caused by gram-negative pathogens [6]. Some common aminoglycoside include apramycin, tobramycin, berkanamycin, ribostamycin, livodomycin, and paromomycin. Aminoacyl site (A-site) ribosomal RNA (rRNA) is the target of these aminoglycoside antibiotics. 16S rRNA constructs have been extensively studied using nuclear magnetic resonance (NMR). It has been showed through Mass spectrometric studies that paromomycin binds most tightly to the A-site rRNA [7].

Binding of paromomycin in the major groove of the A-site RNA displaces the three adenines A1408, A1492 and A1493 toward the minor groove [4]. In the free RNA (absence of paromomycin), the A-site RNA contains an asymmetric loop closed by C1407•G1494 Watson-Crick base pair and by U1406•U1495 and A1408•A1493 non-canonical base pairs. A1492 and A1493 intercalate between the upper and lower stems. Upon paromomycin binding, there is a subtle conformation change in the RNA. The paromomycin binds in the major groove of the A-site RNA, in a binding pocket created by the A1408•A1493 base pair and the bulged 1492 nucleotide.
The conformational change induced by the aminoglycoside binding has implications in the interference of aminoglycoside with translation fidelity. Aminoglycoside antibiotics decrease the dissociation rate of tRNAs from the A-site, they increase the affinity for tRNA-binding to the A site [8]. Recently, it has been showed, that methylation of 16S rRNA serves as resistance against aminoglycosides [9]. Investigation of the mechanism of this methylation is important in antimicrobial therapy and quality control of such antibiotics.
Universal Primers[edit]
The 16SrRNA gene is used for phylogenetic studies[10] as it is highly conserved between different species of bacteria and archaea.[11] Carl Woese pioneered this use of 16S rRNA.[3] In addition to these, mitochondrial and chloroplastic rRNA are also amplified.
The most common primer pair was devised by Weisburg et al.[10] and is currently referred to as 27F and 1492R; however, for some applications shorter amplicons may be necessary for example for 454 sequencing with Titanium chemistry (500-ish reads are ideal) the primer pair 27F-534R covering V1 to V3.[12] Often 8F is used rather than 27F. The two primers are almost identical, but 27F has a M (A or C) instead of a C. AGAGTTTGATCMTGGCTCAG compared with 8F.[13]
| Primer name | Sequence (5'-3') | Reference |
|---|---|---|
| 8F | AGA GTT TGA TCC TGG CTC AG | [14][15] |
| U1492R | GGT TAC CTT GTT ACG ACT T | same as above |
| 928F | TAA AAC TYA AAK GAA TTG ACG GG | [16] |
| 336R | ACT GCT GCS YCC CGT AGG AGT CT | as above |
| 1100F | YAA CGA GCG CAA CCC | |
| 1100R | GGG TTG CGC TCG TTG | |
| 337F | GAC TCC TAC GGG AGG CWG CAG | |
| 907R | CCG TCA ATT CCT TTR AGT TT | |
| 785F | GGA TTA GAT ACC CTG GTA | |
| 805R | GAC TAC CAG GGT ATC TAA TC | |
| 533F | GTG CCA GCM GCC GCG GTA A | |
| 518R | GTA TTA CCG CGG CTG CTG G | |
| 27F | AGA GTT TGA TCM TGG CTC AG | [17] |
| 1492R | CGG TTA CCT TGT TAC GAC TT | as above |
PCR applications[edit]
In addition to highly conserved primer binding sites, 16S rRNA gene sequences contain hypervariable regions that can provide species-specific signature sequences useful for bacterial identification.[18][19] As a result, 16S rRNA gene sequencing has become prevalent in medical microbiology as a rapid and cheap alternative to phenotypic methods of bacterial identification.[20] Although it was originally used to identify bacteria, 16S sequencing was subsequently found to be capable of reclassifying bacteria into completely new species, or even genera.[21][22] It has also been used to describe new species that have never been successfully cultured.[23][24]
16S Ribosomal Databases[edit]
The 16S rRNA gene is used as the standard for classification and identification of microbes, because it is present in most microbes and shows proper changes. Type strains of 16S rRNA gene sequences for most bacteria and archaea are available on public databases such as NCBI. However, the quality of the sequences found on these databases are often not validated. Therefore, secondary databases which collect only 16S rRNA sequences are widely used. The most frequently used databases are listed below:
1) EzTaxon-e. http://eztaxon-e.ezbiocloud.net/ The EzTaxon-e database is an extension of the original EzTaxon database. It contains comprehensive 16S rRNA gene sequences of taxa with valid names as well as sequences of uncultured taxa. EzTaxon-e contains complete hierarchical taxonomic structure (from phylum rank to species rank) for the domain of bacteria and archaea.[25]
2) Ribosomal Database Project. http://rdp.cme.msu.edu/ The Ribosomal Database Project (RDP) is a curated database that offers ribosome data along with related programs and services. The offerings include phylogenetically ordered alignments of ribosomal RNA (rRNA) sequences, derived phylogenetic trees, rRNA secondary structure diagrams and various software packages for handling, analyzing and displaying alignments and trees. The data are available via ftp and electronic mail. Certain analytic services are also provided by the electronic mail server.[26]
3) SILVA. SILVA provides comprehensive, quality checked and regularly updated datasets of aligned small (16S/18S, SSU) and large subunit (23S/28S, LSU) ribosomal RNA (rRNA) sequences for all three domains of life (Bacteria, Archaea and Eukarya).[27]
4) Greengenes. The greengenes web application provides access to the 2011 version of the greengenes 16S rRNA gene sequence alignment for browsing, blasting, probing, and downloading. The data and tools presented by greengenes can assist the researcher in choosing phylogenetically specific probes, interpreting microarray results, and aligning/annotating novel sequences.[28]
References[edit]
- ^ Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution (2000). "Structure of functionally activated small ribosomal subunit at 3.3 angstroms resolution". Cell. 102 (5): 615–23. doi:10.1016/S0092-8674(00)00084-2. PMID 11007480.
{{cite journal}}: External link in|author= - ^ greengenes.lbl.gov - Aligned 16S rDNA data and tools
- ^ a b Woese, C. R.; G. E. Fox (1977-11-01). "Phylogenetic structure of the prokaryotic domain: The primary kingdoms". Proceedings of the National Academy of Sciences. 74 (11): 5088–5090. Bibcode:1977PNAS...74.5088W. doi:10.1073/pnas.74.11.5088. ISSN 0027-8424. PMC 432104. PMID 270744.

- ^ Case RJ, Boucher Y, Dahllöf I, Holmström C, Doolittle WF, Kjelleberg S (January 2007). "Use of 16S rRNA and rpoB Genes as Molecular Markers for Microbial Ecology Studies". Appl. Environ. Microbiol. 73 (1): 278–88. doi:10.1128/AEM.01177-06. PMC 1797146. PMID 17071787.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Fourmy, Dominique (1998). "Paromomycin Binding Induced a Local Conformational Change in the A-site of 16 S rRNA". Journal of Molecular Biology. 277 (2): 333–345. doi:10.1006/jmbi.1997.1551. PMID 9514734.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barrett, Julia. "Aminoglycosides". Gale Encyclopedia of Medicine. Retrieved 2002.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Griffey, Richard (August 1999). "Determinants of amino glycoside-binding specificity for rRNA by using mass spectrometry". Prod. Natl. Acad. Sci. 96 (18): 10129–10133. doi:10.1073/pnas.96.18.10129. PMC 17854. PMID 10468574.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Fourmy, Dominique (March 1998). "Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA". J. Mol. Biol. 277 (2): 347–362. doi:10.1006/jmbi.1997.1552. PMID 9514735.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ Arakawa, Yoshichika (July 2007). "16S Ribosomal RNA Methylation: Emerging Resistance Mechanism against Aminoglycosides". Antimicrobial Resistance (45): 88–94.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: date and year (link) - ^ a b Weisburg WG, Barns SM, Pelletier DA, Lane DJ (January 1991). "16S ribosomal DNA amplification for phylogenetic study". J Bacteriol. 173 (2): 697–703. doi:10.1128/jb.173.2.697-703.1991. PMC 207061. PMID 1987160.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Coenye T, Vandamme P (November 2003). "Intragenomic heterogeneity between multiple 16S ribosomal RNA operons in sequenced bacterial genomes". FEMS Microbiol. Lett. 228 (1): 45–49. doi:10.1016/S0378-1097(03)00717-1. PMID 14612235.
{{cite journal}}: CS1 maint: date and year (link) - ^ http://www.hmpdacc.org/tools_protocols.php#sequencing
- ^ http://www.lutzonilab.net/primers/page604.shtml
- ^ Eden PA, Schmidt TM, Blakemore RP, Pace NR (1991). "Phylogenetic Analysis of Aquaspirillum magnetotacticum Using Polymerase Chain Reaction-Amplified 16S rRNA-Specific DNA". Int J Syst Bacteriol. 41 (2): 324–325. doi:10.1099/00207713-41-2-324. PMID 1854644.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Universal Bacterial Identification by PCR and DNA Sequencing of 16S rRNA Gene. PCR for Clinical Microbiology, 2010, Part 3, 209-214
- ^ Weidner S, Arnold W, Pühler A (1996). "Diversity of uncultured microorganisms associated with the seagrass Halophila stipulacea estimated by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes" (PDF). Appl Env Microbiol. 62 (3): 766–71. doi:10.1128/aem.62.3.766-771.1996. PMC 167844. PMID 8975607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jiang, H.; Dong, H.; Zhang, G.; Yu, B.; Chapman, L. R.; Fields, M. W. (2006). "Microbial Diversity in Water and Sediment of Lake Chaka, an Athalassohaline Lake in Northwestern China". Applied and Environmental Microbiology. 72 (6): 3832–3845. doi:10.1128/AEM.02869-05. PMC 1489620. PMID 16751487.
- ^ Pereira, Filipe; Carneiro, João; Matthiesen, Rune; Van Asch, Barbara; Pinto, Nádia; Gusmão, Leonor; Amorim, António (4 October 2010). "Identification of species by multiplex analysis of variable-length sequences". Nucleic Acids Research. 38 (22): e203. doi:10.1093/nar/gkq865. PMC 3001097. PMID 20923781.
- ^ Kolbert, Christopher P.; Persing, David H. (June 1999). "Ribosomal DNA sequencing as a tool for identification of bacterial pathogens". Current Opinion in Microbiology. 2 (3): 299–305. doi:10.1016/S1369-5274(99)80052-6. PMID 10383862.
- ^ J. E. Clarridge III (2004). "Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases". Clin Microbiol Rev. 17 (4): 840–862. doi:10.1128/CMR.17.4.840-862.2004. PMC 523561. PMID 15489351.
- ^ Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991). "16S ribosomal DNA amplification for phylogenetic study". J Bacteriol. 173 (2): 697–703. doi:10.1128/jb.173.2.697-703.1991. PMC 207061. PMID 1987160.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brett P J, DeShazer D, Woods DE (1998). "Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species". Int J Syst Bacteriol. 48: 317–320. doi:10.1099/00207713-48-1-317. PMID 9542103.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schmidt TM, Relman DA (1994). "Phylogenetic identification of uncultured pathogens using ribosomal RNA sequences". Methods Enzymol. Methods in Enzymology. 235: 205–22. doi:10.1016/0076-6879(94)35142-2. ISBN 978-0-12-182136-4. PMID 7520119.
- ^ Gray JP, Herwig RP (1996). "Phylogenetic analysis of the bacterial communities in marine sediments". Appl Environ Microbiol. 62 (11): 4049–59. doi:10.1128/aem.62.11.4049-4059.1996. PMC 168226. PMID 8899989.
- ^ Chun, J., Lee, J.-H., Jung, Y., Kim, M., Kim, S., Kim, B. K. & Lim, Y. W. (2007). EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57, 2259-2261.
- ^ Larsen N, Olsen GJ, Maidak BL, McCaughey MJ, Overbeek R, Macke TJ, Marsh TL, Woese CR.(1993) The ribosomal database project. Nucleic Acids Res. Jul 1;21(13):3021-3.
- ^ Elmar Pruesse, Christian Quast, Katrin Knittel, Bernhard M. Fuchs, Wolfgang Ludwig, Jörg Peplies, Frank Oliver Glöckner (2007) Nucleic Acids Res. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. December; 35(21): 7188–7196.
- ^ DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl Environ Microbiol 72:5069-72.

