Methanol toxicity
| Methanol toxicity | |
|---|---|
| Other names | Methanol poisoning, methanol overdose |
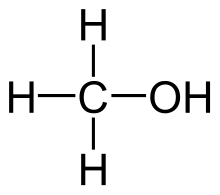 | |
| Molecular structure of methanol | |
| Specialty | Emergency medicine |
| Symptoms | Decreased level of consciousness, poor coordination, vomiting, abdominal pain, specific smell on the breath[1][2] |
| Complications | Blindness, kidney failure[1] |
| Causes | Methanol (such as found in windshield washer fluid)[1][2] |
| Diagnostic method | Blood acidosis, increased osmol gap, methanol blood level[1][2] |
| Differential diagnosis | Infections, exposure to other toxic alcohols, serotonin syndrome, diabetic ketoacidosis[2] |
| Prevention | Consuming safe alcoholic beverages |
| Treatment | Antidote, hemodialysis[2] |
| Medication | Fomepizole, ethanol[2] |
| Prognosis | Good with early treatment[1] |
| Frequency | 1,700 cases per year (US)[3] |
Methanol toxicity (also methanol poisoning) is poisoning from methanol, characteristically via ingestion.[1] Symptoms may include an altered/decreased level of consciousness, poor or no coordination, vomiting, abdominal pain, and a specific smell on the breath.[1][2] Decreased vision may start as early as twelve hours after exposure.[2] Long-term outcomes may include blindness and kidney failure.[1] Blindness may occur after drinking as little as 10 mL; death may occur after drinking quantities over 15 mL (median 100 mL, varies depending on body weight).[1][4]
Methanol poisoning most commonly occurs following the drinking of windshield washer fluid.[2] This may be accidental or as part of an attempted suicide. Toxicity may also rarely occur through extensive skin exposure or breathing in fumes.[1] When the body breaks down methanol it results in the creation of metabolite byproducts such as formaldehyde, formic acid, and formate which cause much of the toxicity.[2] The diagnosis may be suspected when there is acidosis or an increased osmol gap and confirmed by directly measuring blood levels.[1][2] Other conditions that can produce similar symptoms include infections, exposure to other toxic alcohols, serotonin syndrome, and diabetic ketoacidosis.[2]
Early treatment increases the chance of a good outcome. Treatment consists of stabilizing the person and using an antidote. The preferred antidote is fomepizole, with ethanol used if this is not available. Hemodialysis may also be used in those where there is organ damage or a high degree of acidosis. Other treatments may include sodium bicarbonate, folate, and thiamine.[2]
Outbreaks of methanol ingestion have occurred due to contamination of drinking alcohol. This is more common in the developing world.[2] In 2013 more than 1700 cases occurred in the United States. Those affected are usually adults and males.[3] Toxicity to methanol has been described as early as 1856.[5]
Signs and symptoms
[edit]The initial symptoms of methanol intoxication include central nervous system depression, headache, dizziness, nausea, lack of coordination, and confusion. Sufficiently large doses cause unconsciousness and death. The initial symptoms of methanol exposure are usually less severe than the symptoms from the ingestion of a similar quantity of ethanol.[6] Once the initial symptoms have passed, a second set of symptoms arises, from 10 to as many as 30 hours after the initial exposure, that may include blurring, photophobia, snowstorm vision or complete loss of vision, acidosis, and putaminal hemorrhages, an uncommon but serious complication.[7][8] These symptoms result from the accumulation of toxic levels of formate in the blood, and may progress to death by respiratory failure. Physical examination may show tachypnea, and eye examination may show dilated pupils with hyperemia of the optic disc and retinal edema.
Cause
[edit]Methanol has a moderate to high toxicity in humans. As little as 10 mL of pure methanol when drunk is metabolized into formic acid, which can cause permanent blindness by destruction of the optic nerve. 15 mL is potentially fatal,[1] although the median lethal dose is typically 100 mL (3.4 fl oz) (i.e. 1–2 mL/kg body weight of pure methanol).[4] Reference dose for methanol is 0.5 mg/kg/day.[9]
Moonshine distilled from fermented fruit
[edit]Although methanol is not produced in toxic amounts by fermentation of sugars from grain starches,[10] it is a major occurrence in fruit spirits.[11] However, in modern times, reducing methanol with the absorption of a molecular sieve is a practical method for production.[12]
Surrogate alcohol
[edit]Because of its similarities in both appearance and odor to ethanol (the alcohol in beverages) or isopropyl alcohol, it is difficult to differentiate between the three.[13] As a result, ethanol is sometimes denatured (adulterated), and made poisonous, by the addition of methanol. The result is known as methylated spirit, "meths" (British use) or "metho" (Australian slang). [14]
This is not to be confused with "meth", a common abbreviation for methamphetamine and for methadone in Britain and the United States.[citation needed]
Despite its poisonous content, denatured alcohol is sometimes consumed as a surrogate alcohol.[citation needed]
Mechanism
[edit]Methanol is toxic by two mechanisms. First, methanol (whether it enters the body by ingestion, inhalation, or absorption through the skin) can be fatal due to its CNS depressant properties in the same manner as ethanol poisoning. Second, in a process of toxication, it is metabolized to formic acid (which is present as the formate ion) via formaldehyde in a process initiated by the enzyme alcohol dehydrogenase in the liver.[15] Methanol is converted to formaldehyde via alcohol dehydrogenase and formaldehyde is converted to formic acid (formate) via aldehyde dehydrogenase. The conversion to formate via ALDH proceeds completely, with no detectable formaldehyde remaining.[16] Formate is toxic because it inhibits mitochondrial cytochrome c oxidase, causing hypoxia at the cellular level, and metabolic acidosis, among a variety of other metabolic disturbances.[17]
Treatment
[edit]Methanol poisoning can be treated with fomepizole, or if unavailable, ethanol may be used.[15][18][19] Both drugs act to reduce the action of alcohol dehydrogenase on methanol by means of competitive inhibition. Ethanol, the active ingredient in alcoholic beverages, acts as a competitive inhibitor by more effectively binding and saturating the alcohol dehydrogenase enzyme in the liver, thus blocking the binding of methanol. Methanol is excreted by the kidneys without being converted into the toxic metabolites formaldehyde and formic acid. Alcohol dehydrogenase instead enzymatically converts ethanol to acetaldehyde, a less toxic organic molecule.[15][20] Additional treatment may include sodium bicarbonate for metabolic acidosis, and hemodialysis or hemodiafiltration to remove methanol and formate from the blood.[15] Folinic acid or folic acid is also administered to enhance the metabolism of formate.[15]
History
[edit]There are cases of methanol resistance, such as that of Mike Malloy, whom someone tried and failed to poison with methanol in the early 1930s.[21]
In December 2016, 78 people died in Irkutsk, Russia from methanol poisoning after ingesting a counterfeit body lotion that was primarily methanol rather than ethanol as labeled. The body lotion, before the event, had been used as a cheap substitute for vodka by the impoverished people in the region despite warnings on the lotion's bottles that it was not safe for drinking and long-standing problems with alcohol poisoning across the country.[22]
During the COVID-19 pandemic, Iranian media reported that nearly 300 people had died and over a thousand became ill due to methanol poisoning in the belief that drinking methanol could help with the disease.[23] In the United States, the Food and Drug Administration discovered that several brands of hand sanitizer manufactured in Mexico during the pandemic contained methanol, and urged the public to avoid using the affected products.[24]
In November 2024, six foreign tourists, comprising two Australian teenagers, two Danish women, a British lawyer, and an American tourist, died of suspected methanol poisoning after consuming contaminated alcohol at the Nana Backpackers Hostel in Vang Vieng, Laos. The tragedy, which also hospitalized several others, prompted Laotian authorities to detain eight hostel staff members and launch an investigation into the source of the contamination. Governments, including Australia, updated travel advisories, warning citizens about the dangers of consuming local alcohol in Southeast Asia. The incident significantly affected Laos's tourism-dependent economy, as travelers canceled trips and raised concerns over safety standards in the country. Tourism, a critical sector for Laos, faced losses as international confidence waned, prompting calls for stricter regulations on alcohol production and improved enforcement to restore trust.[25][26][27]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l Kruse JA (October 2012). "Methanol and ethylene glycol intoxication". Critical Care Clinics. 28 (4): 661–711. doi:10.1016/j.ccc.2012.07.002. PMID 22998995.
- ^ a b c d e f g h i j k l m n Beauchamp GA, Valento M (September 2016). "Toxic Alcohol Ingestion: Prompt Recognition And Management In The Emergency Department". Emergency Medicine Practice. 18 (9): 1–20. PMID 27538060.
- ^ a b Ferri FF (2016). Ferri's Clinical Advisor 2017: 5 Books in 1. Elsevier Health Sciences. p. 794. ISBN 9780323448383. Archived from the original on 2017-09-08.
- ^ a b "Methanol Poisoning Overview". Antizol. Archived from the original on 5 October 2011.
- ^ Clary JJ (2013). The Toxicology of Methanol. John Wiley & Sons. p. 3.4.1. ISBN 9781118353103. Archived from the original on 2017-09-08.
- ^ National Institute for Occupational Safety and Health (22 August 2008). "The Emergency Response Safety and Health Database: Methanol". Archived from the original on 23 April 2009. Retrieved 17 March 2009.
- ^ Jafarizadeh, A., Homaie, M., Abdollahi, M., & Niyousha, M. (2023). Time course study of optical coherence tomography angiography in patients with methanol-induced optic neuropathy. BMC ophthalmology, 23(1), 178. https://doi.org/10.1186/s12886-023-02937-x
- ^ Permpalung N, Cheungpasitporn W, Chongnarungsin D, Hodgdon TM (Oct 2013). "Bilateral putaminal hemorrhages: serious complication of methanol intoxication". N Am J Med Sci. 5 (10): 623–4. doi:10.4103/1947-2714.120804. PMC 3842708. PMID 24350079.
- ^ Methanol (CASRN 67-56-1) Archived 2012-12-05 at the Wayback Machine
- ^ "Distillation: Some Purity Considerations". Moonshine Still. Retrieved 5 May 2015.
- ^ Blumenthal, P; Steger, MC; Einfalt, D; Rieke-Zapp, J; Quintanilla Bellucci, A; Sommerfeld, K; Schwarz, S; Lachenmeier, DW (28 April 2021). "Methanol Mitigation during Manufacturing of Fruit Spirits with Special Consideration of Novel Coffee Cherry Spirits". Molecules. 26 (9): 2585. doi:10.3390/molecules26092585. PMC 8125215. PMID 33925245.
- ^ Hui-Ling Ma; Xiu-Ping Yang; Ying Zuo (15 April 2006). "Study on Method of Decreasing Methanol in Apple Pomace Spirit". Food Science. 27 (4): 138–142.
- ^ Wade, Leroy G. "Physical properties of alcohols". Britannica. Retrieved 2024-08-18.
- ^ https://healthinfonet.ecu.edu.au/key-resources/resources/27191/?title=Metho++methylated+spirits+++It+s+not+a+drink+&contenttypeid=1&contentid=27191_1
- ^ a b c d e Schep LJ, Slaughter RJ, Vale JA, Beasley DM (2009). "A seaman with blindness and confusion". BMJ. 339: b3929. doi:10.1136/bmj.b3929. PMID 19793790. S2CID 6367081. Archived from the original on 2009-10-08.
- ^ McMartin KE, Martin-Amat G, Noker PE, Tephly TR (1979). "Lack of a role for formaldehyde in methanol poisoning in the monkey". Biochem. Pharmacol. 28 (5): 645–9. doi:10.1016/0006-2952(79)90149-7. PMID 109089.
- ^ Liesivuori J, Savolainen H (September 1991). "Methanol and formic acid toxicity: biochemical mechanisms". Pharmacol. Toxicol. 69 (3): 157–63. doi:10.1111/j.1600-0773.1991.tb01290.x. PMID 1665561.
- ^ Casavant MJ (January 2001). "Fomepizole in the treatment of poisoning". Pediatrics. 107 (1): 170. doi:10.1542/peds.107.1.170. PMID 11134450. Archived from the original on 2005-06-29.
- ^ Brent J (May 2009). "Fomepizole for ethylene glycol and methanol poisoning". The New England Journal of Medicine. 360 (21): 2216–2223. doi:10.1056/NEJMct0806112. PMID 19458366.
- ^ Voet D, Voet JG, Pratt CW (2008). Fundamentals of Biochemistry: Life at the Molecular Level (5th ed.). Hoboken, NJ: Wiley.
- ^ Blum D (2010). The Poisoner's Handbook: murder and the birth of forensic medicine in Jazz Age New York. New York: Penguin Books. p. 231. ISBN 978-0-14-311882-4.
- ^ Isachenkov V (19 December 2016). "Alcohol poisoning death toll in Russian city rises to 49". Associated Press. Archived from the original on 20 December 2016. Retrieved 19 December 2016.
- ^ Karimi, Nasser; Gambrell, Jon (27 March 2020). "In Iran, false belief a poison fights virus kills hundreds". Associated Press. Archived from the original on 27 March 2020. Retrieved 27 March 2020.
- ^ "FDA Updates on Hand Sanitizers with Methanol". U.S. Food and Drug Administration. Retrieved 28 July 2020.
- ^ Yeung, Jessie (21 November 2024). "Australian backpackers among six dead in suspected methanol poisoning in Laos". CNN. Retrieved 26 November 2024.
- ^ Davies, Caroline; Ferguson, Donna (22 November 2024). "British lawyer among six to die in suspected methanol poisoning in Laos". The Guardian. Retrieved 26 November 2024.
- ^ "Australia warns travelers after methanol poisoning deaths in Laos". news.com.au. 24 November 2024. Retrieved 26 November 2024.
