Orotic acid: Difference between revisions
Updating {{drugbox}} (changes to watched fields - added verified revid) per Chem/Drugbox validation (report errors or bugs) |
|||
| Line 2: | Line 2: | ||
{{drugbox |
{{drugbox |
||
| Watchedfields = changed |
|||
| verifiedrevid = 265738220 |
|||
| IUPAC_name = 1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylic acid |
| IUPAC_name = 1,2,3,6-tetrahydro-2,6-dioxo-4-pyrimidinecarboxylic acid |
||
| image = Orotic-acid-2D-skeletal.png |
| image = Orotic-acid-2D-skeletal.png |
||
Revision as of 17:01, 5 November 2009
This article needs additional citations for verification. (October 2007) |
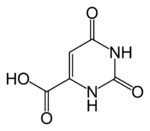 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.563 |
| Chemical and physical data | |
| Formula | C5H4N2O4 |
| Molar mass | 156.10 g/mol g·mol−1 |
| (verify) | |
Orotic acid is a heterocyclic compound and an acid; it is also known as pyrimidinecarboxylic acid. It was once believed to be part of the vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin but is instead manufactured in the body by intestinal flora.[citation needed]
Its salts, known as orotates, are sometimes used as mineral carriers in some dietary supplements, to increase their bioavailability. Lithium orotate is the most frequently used in this manner.
Orotic Acid diseases
A buildup of orotic acid can lead to orotic aciduria.
In ornithine transcarbamoylase deficiency, a disorder of the urea cycle, excess carbamoyl phospate is converted into orotic acid. This typically leads to increased urinary orotic acid excretion.
See also
References
- Orotic Acid antagonist: "6-Uracilsulfonic Acid, a Sulronic Acid Analog of Orotic Acid" Sheldon B. Greenbaum, J. Am. Chem. Soc., 1954, 76 (23), pp 6052–6054
External links
- Orotic+Acid at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Overview at ggc.org
- Overview at greatvistachemicals.com
- Overview of Potential Metabolic Antagonists
