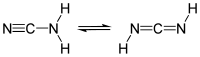Cyanamide: Difference between revisions
Script assisted update of chemical identifiers from ChemSpider for the Chem/Drugbox validation project. |
Updating {{chembox}} (no changed fields - added verified revid) per Chem/Drugbox validation (report errors or bugs) |
||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| ⚫ | |||
| Watchedfields = changed |
|||
| ⚫ | |||
| ImageFileL1 = Cyanamide.svg |
| ImageFileL1 = Cyanamide.svg |
||
| ImageSizeL1 = 200px |
| ImageSizeL1 = 200px |
||
Revision as of 11:01, 27 January 2010
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Cyanamide,
aminomethanenitrile | |||
| Other names
Amidocyanogen, carbamonitrile, carbimide, carbodiimide, cyanoamine, cyanoazane, N-cyanoamine, cyanogenamide, cyanogen nitride, hydrogen cyanamide
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.006.358 | ||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 2811 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2N2 | |||
| Molar mass | 42.040 g/mol | ||
| Appearance | Crystalline solid | ||
| Density | 1.28 g/cm3 | ||
| Melting point | 44 °C | ||
| Boiling point | 260 °C (decomp.) 83 °C at 6.7 Pa 140 °C at 2.5 kPa | ||
| 85 g/100 ml (25 °C) | |||
| Solubility in organic solvents | soluble | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 141 °C | ||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
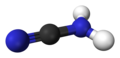
Cyanamide is an organic compound with the formula CN2H2. This white solid is widely used in synthetic organic chemistry as well as in agriculture. It is also used as an alcohol deterent drug in Canada, Europe and Japan, but not in the United States. The molecule features a nitrile group attached to an amino group. Although it is similar in structure to hydrogen cyanide, it is not as toxic. Derivative of this compound are also referred to as cyanamides, the most common being calcium cyanamide (CaCN2).
Production, reactions, uses
Cyanamide is produced by hydrolysis of calcium cyanamide, which in turn is prepared from calcium carbide via the Frank-Caro process.
- CaCN2 + H2O + CO2 → CaCO3 + H2NCN
The conversion is conducted on slurries, consequently most commercial cyanamide is sold as an aqueous solution.
The main reaction exhibited by cyanamide involves additions of protic reagents. Water, hydrogen sulfide, and hydrogen selenide add to give urea, thiourea, and selenourea, respectively:
- H2NCN + H2E → H2NC(E)NH2 (E = O, S, Se)
Thus, cyanamide is a dehydration agent and thus can induce condensation reactions. Alcohols, thiols, and amines react analogously to give alkylisoureas, "pseudothioureas," and guanidines. The anti-ulcer drug cimetidine is generated using such reactivity. Related reactions exploit the bifunctionality of cyanamide to give heterocycles, and this latter reactivity is the basis of several pharmaceutical syntheses such as the aminopyrimidine imatinib) and agrichemicals Amitrol (3-Amino-1,2,4-triazole) and Hexazinone. The hair-loss treatment Minoxidil and the anthelmintic (worm-killing) drugs Albendazole, Flubendazole, and Mebendazole feature 2-aminoimidazole substructures derived from cyanamide.[1]
Environmental aspects
Cyanamide degrades via hydrolysis to urea, an excellent fertilizer. Microorganisms, e.g. the bacterium Myrothecium verrucaria, accelerate this process utilizing the enzyme cyanamide hydratase.
Safety
Cyanamide has a modest toxicity in humans.[2] Workplace exposure to hydrogen cyanamide sprays or exposure in people living in the vicinity of spraying have been reported as causing respiratory irritation, contact dermatitis, headache, and gastrointestinal symptoms of nausea, vomiting, or diarrhea.[2]
References
- ^ Thomas Güuthner,Bernd Mertschenk, "Cyanamides" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006.
- ^ a b Schep L, Temple W, Beasley M (2009). "The adverse effects of hydrogen cyanamide on human health: an evaluation of inquiries to the New Zealand National Poisons Centre". Clinical Toxicology (Philadelphia, Pa.). 472 (1): 58–60. doi:10.1080/15563650802459254. PMID 18951270.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- International Chemical Safety Card 0424
- NIOSH Pocket Guide to Chemical Hazards. "#0160". National Institute for Occupational Safety and Health (NIOSH).
- OSHA guideline for cyanamide