RDX: Difference between revisions
→Usage: added Trade name |
|||
| Line 302: | Line 302: | ||
* [http://www.adi-limited.com/2-01-050-030-000.html ADI Limited (Australia)] |
* [http://www.adi-limited.com/2-01-050-030-000.html ADI Limited (Australia)] |
||
* [http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@na+@rel+cyclonite NLM Hazardous Substances Databank (USA) – Cyclonite (RDX)] |
* [http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@na+@rel+cyclonite NLM Hazardous Substances Databank (USA) – Cyclonite (RDX)] |
||
*{{Citation |
|||
|last= GlobalSecurity.org |
|||
|url= http://www.globalsecurity.org/military/systems/munitions/explosives-compositions.htm |
|||
|title= Explosives - Compositions |
|||
|publisher= GlobalSecurity.org |
|||
|location= Alexandria, VA |
|||
|accessdate= September 1, 2010 |
|||
|doi=}} |
|||
{{Polytonic|}} |
{{Polytonic|}} |
||
Revision as of 16:15, 1 September 2010
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3,5-Trinitroperhydro-1,3,5-triazine
| |||
| Other names
RDX
1,3,5-Trinitro-1,3,5-triazacyclohexane 1,3,5-Trinitrohexahydro-s-triazine cyclonite, hexogen, Cyclotrimethylenetrinitramine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ECHA InfoCard | 100.004.092 | ||
PubChem CID
|
|||
| UN number | 0072, 0391, 0483 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| C3H6N6O6 | |||
| Molar mass | 222.117 g·mol−1 | ||
| Appearance | Colorless crystals | ||
| Density | 1.82 g/cm3 | ||
| Melting point | 205.5 °C (401.9 °F; 478.6 K) | ||
| Boiling point | 234 °C (453 °F; 507 K) | ||
| Explosive data | |||
| Shock sensitivity | Low | ||
| Friction sensitivity | Low | ||
| RE factor | 1.60 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Explosive | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
RDX, also known less commonly as cyclonite, hexogen (particularly in German and German-influenced languages), and T4, and chemically as Cyclotrimethylenetrinitramine, is an explosive nitroamine widely used in military and industrial applications. Nomenclature variants include cyclotrimethylene-trinitramine and cyclotrimethylene trinitramine.
In its pure, synthesized state RDX is a white, crystalline solid. As an explosive, it is usually used in mixtures with other explosives and plasticizers, phlegmatizers or desensitizers. It is stable in storage and is considered one of the most powerful and brisant of the military high explosives.[1]
Name
RDX is also known, but less commonly, as cyclonite, hexogen (particularly in German and German-influenced languages), T4 and chemically as cyclotrimethylenetrinitramine. Tenney L Davis, writing in the USA in 1943, stated it was generally known in the USA as cyclonite; the Germans called it Hexogen, the Italians T4.[2] In the 1930s, the Royal Arsenal, Woolwich, started investigating cyclonite as an explosive to use against German U-boats that were being built with thicker hulls. Britain wanted an explosive that was more powerful than TNT. For security reasons, Britain termed cyclonite as "Research Department Explosive" (R. D. X.).[3] The term RDX appeared in the United States in 1946, but the name RDX is given without explanation.[4] The first public reference in the United Kingdom to the name RDX, or R.D.X. to use the official title, appeared in 1948; its authors were the Managing Chemist, ROF Bridgwater, the Chemical Research and Development Department, Woolwich, and the Director of Royal Ordnance Factories, Explosives; again, it was referred to as simply RDX.[5]
Usage
RDX was widely used during World War II, often in explosive mixtures with TNT such as Torpex, Composition B, Cyclotols, and H6. RDX was used in one of the first plastic explosives. RDX is believed to have been used in many bomb plots including terrorist plots. The bombs used in the "Dambusters Raid" contained 6,600 pounds of Torpex.[6]
RDX forms the base for a number of common military explosives:
- Composition A: Granular explosive consisting of RDX and plasticizing wax. Such as, composition A-5 (RDX coated with 1.5% stearic acid) and composition A-3 (91% RDX coated with 9% wax)
- Composition B: castable mixtures of RDX and TNT
- Composition C: The original composition C was used in World War II, but there have been subsequent variations including C-2, C-3, and C-4. C-4 consists of RDX (91%), a plasticizer (which can be dioctyl adipate {DOA}, diethylhexyl, or dioctyl sebacate) (5.3%), a binder, which is usually polyisobutylene (2.1%), SAE 10 non-detergent motor oil (1.6%).
- Composition CH-6: 97.5% RDX, 1.5% calcium stearate, 0.5% polyisobutylene, and 0.5% graphite.
- Composition D:
- Cyclotol:
- HBX: castable mixtures of RDX, TNT, powdered aluminium, and D-2 wax with calcium chloride
- H-6: castable mixture of RDX, TNT, powdered aluminum, and paraffin wax
- Semtex: (Trade name): plastic demolition explosives containing RDX and PETN as major energetic components
- Torpex: 42% RDX, 40% TNT and 18% powdered aluminium
- PBX: RDX is also used as a major component of many plastic bonded explosives (PBX). RDX-based PBX's typically consist of RDX and a polymer/co-polymer binder. Examples of RDX-based PBX formulations include, but are not limited to: PBX-9007, PBX-9010, PBX-9205, PBX-9407, PBX-9604, PBXN-106, PBXN-3, PBXN-6, PBXN-10, PBXN-201, PBX-0280, PBX Type I, PBXC-116, PBXAF-108, etc.
Outside of military applications, RDX is also used in controlled demolition to raze structures. The demolition of the Jamestown Bridge in the U.S. state of Rhode Island is one example where RDX shaped charges were used to remove the span.
Properties
The velocity of detonation of RDX at a density of 1.76 g/cm³ is 8750 m/s.
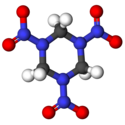
It is a colourless solid, of maximum theoretical density 1.82 g/cm³. It is obtained by reacting concentrated nitric acid with hexamine.[7]
- (CH2)6N4 + 10HNO3 → (CH2-N-NO2)3 + 3CH2(ONO2)2 + NH4NO3 + 3H2O
It is a heterocycle and has the molecular shape of a ring. It starts to decompose at about 170 °C and melts at 204 °C. Its structural formula is: hexahydro-1,3,5-trinitro-1,3,5-triazine or (CH2-N-NO2)3.
At room temperature, it is very stable. It burns rather than explodes and detonates only with a detonator, being unaffected even by small arms fire. It is less sensitive than pentaerythritol tetranitrate (PETN). However, it is very sensitive when crystallized, below −4 °C. Under normal conditions, RDX has a figure of insensitivity of exactly 80 (as this is the reference point).
RDX sublimes in vacuum, which limits its use in pyrotechnic fasteners for spacecraft.
History
The discovery of RDX dates from 1898 when Georg Friedrich Henning obtained a German patent (patent No. 104280) for its manufacture, by nitrating hexamine nitrate (hexamethylenetetramine nitrate) with concentrated nitric acid.[8] In this 1898 patent, its properties as a medical compound were mentioned; however, three futher German patents obtained by Henning in 1916 proposed its use in smokeless propellants.[8] Research and development findings were not published further until Edmund von Herz,[9] described as an Austrian and later a German citizen, obtained a British patent in 1921[10] and a U.S. patent in 1922.[11] Both patent claims were initiated in Austria; and described the manufacture of RDX by nitrating hexamethylenetetramine.[10][11] The British patent claims included the manufacture of RDX by nitration, its use with or without other explosives, and its use as a bursting charge and as a initiator.[10] The U.S. patent claim was for the use of a hollow explosive device containing RDX and a detonator cap containing RDX.[11]
RDX was used by both sides in World War II.
UK
In the United Kingdom (UK) RDX was manufactured from 1933 by the Research Department in a pilot plant at the Royal Arsenal in Woolwich, London; a larger pilot plant being built at the RGPF Waltham Abbey just outside London in 1939.[12][13] In 1939 a twin-unit industrial-scale plant was designed to be installed at a new 700 acres (280 ha) site, ROF Bridgwater, away from London; and production of RDX started at Bridgwater on one unit in August 1941.[12][14] The ROF Bridgwater plant brought in ammonia and methanol as raw materials: the methanol was converted to formaldehyde and some of the ammonia converted to nitric acid, which was concentrated for RDX production.[15] The rest of the ammonia was reacted with formaldehyde to produce hexamine. RDX was produced by continually adding hexamine and concentrated nitric acid to a cooled mixture of hexamine and nitric acid in the nitrator.[15] The RDX was purified and processed for its intended use; and recovery and reuse of some methanol and nitric acid was also carried out.[15] The hexamine-nitration and RDX purification plants were duplicated (i.e. twin-unit) to provide some insurance against loss of production due to fire, explosion or air attack.[12]
The United Kingdom and British Empire were fighting without allies against Nazi Germany until the end of 1941 and had to be self-sufficient. At that time (1941), the UK had of capacity to produce 70 long tons (71 t) (160,000 lb) of RDX per week; both Canada, an allied country and former self-governing dominion of the British Empire, and the United States were looked upon to supply ammunition and explosives, including RDX.[16] By 1942 the Royal Air Force's annual requirement was forecast to be 52,000 long tons (53,000 t) of RDX, much of which came from North America (Canada and the U.S.).[14]
Canada
A different method of production to the Woolwich process, was found and used in Canada, possibly at the McGill University Department of Chemistry. This was based on reacting paraformaldehyde and ammonium nitrate in acetic anhydride.[17] A UK patent application was made by Robert Walter Schiessler, Pennsylvania State College and James Hamilton Ross, at McGill, Canada, in May 1942 and the UK patent was issued in December 1947.[18] Gilman states that the same method of production had been independently discovered by Ebele in Germany prior to Schiessler and Ross, but that this was not known by the Allies.[17][8] Urbanski provides details of five methods of production: this is listed as the fourth method.[8]
UK, U.S. and Canadian production and development
At the beginning of the 1940s, the major U.S. explosive manufacturers, E. I. du Pont de Nemours & Company and Hercules had several decades of experience of manufacturing Trinitrotoluene (TNT) and had no wish to experiment with new explosives; a view also held by the U.S. Army Ordnance, who proposed to continue using TNT.[19] RDX had been tested by Picatinny Arsenal in 1929 and it was regarded as too expensive and too sensitive.[16] The Navy proposed to continue using ammonium picrate.[19] In contrast, the view that new explosives were unnecessary was not shared by the National Defense Research Committee (NDRC), who had visited The Royal Arsenal, Woolwich.[19] James B. Conant, chairman of Division B, wished to involve academic research into this area. Conant therefore set up an Experimental Explosives Research Laboratory at the Bureau of Mines, Bruceton, Pennsylania using direct Office of Scientific Research and Development (OSRD) funding.[16]
In 1941, the UK's Tizard Mission visited the U.S. Army and Navy departments and part of the information handed over included details of the "Woolwich" method of manufacture of RDX and its stabilisation by mixing it with beeswax.[16] The UK was asking that the U.S. and Canada, combined, supply 220 long tons (220 t) (490,000 lb) of RDX per day.[16] A decision was taken by William H. P. Blandy, Chief of the Bureau of Ordnance to adopt RDX for use in mines and torpedoes.[16] Given the immediate need for RDX, the U.S. Army Ordnance, at Blandy's request, built a plant that just copied the equipment and process used at Woolwich. The result was the Wabash River Ordinance Works run by E. I. du Pont de Nemours & Company.[20] This works had the largest nitric acid plant in the world, at that time.[16] The Woolwich process was expensive; it needed 11 pounds of strong nitric acid for every pound of RDX.[21]
By early 1941, the NDRC was researching new processes.[21] At least, three laboratories, with no previous explosive experience, were tasked to work on the development of RDX; they were based at Cornell, Michigan and Penn State universities.[16][22] Werner Emmanuel Bachmann, from Michigan, successfully developed the "combination process" by combining the Canadian process with direct nitration.[16] This process which required large quantities of acetic anhydride instead of nitric acid in the old British "Woolwich process".
Vast increases in production of RDX could not continued to rely on the use of the beeswax, first used in the Woolwich process, to desensitize the RDX. A substitute based on petroleum was developed at the Bruceton Explosives Research Laboratory.[16]
Bachmann process
The NDRC tasked three companies to develop pilot plants. They were the Western Cartridge Company, E. I. du Pont de Nemours & Company and Tennessee Eastman Company, part of Eastman Kodak.[16] The Eastman Chemical Company (TEC), Kingsport, Tennessee, a leading manufacturer of acetic anhydride, successfully developed a continuous-flow manufacturing process for RDX. RDX was crucial to the war effort and the current batch-production process could not keep up. In February 1942, TEC built the Wexler Bend pilot plant and began producing small amounts of RDX. This led to the U.S. government authorizing TEC to design and build Holston Ordnance Works (H.O.W.) in June 1942. By April 1943, RDX was being manufactured there.[23] At the end of 1944, the Holston plant and the Wabash River Ordinance Works (which used the Woolwich process) were making 50 million pounds of Composition B per month.[24]
The U.S. Bachmann process for RDX was found to be richer in HMX than the United Kingdom's RDX. This later led to a RDX plant using the Bachmann process being set up at ROF Bridgwater in 1955, to produce both RDX and HMX.
Terrorism
Ahmed Ressam, the al-Qaeda Millenium Bomber, used a small quantity of RDX as one of the components in the explosives that he prepared to bomb Los Angeles International Airport on New Year's Eve 1999/2000; the combined explosives could have produced a blast forty times greater than that of a devastating car bomb.[25][26]
RDX was main component used for the 2006 Mumbai train bombings.[27] It was also believed to be the explosive in the 2010 Moscow Metro bombings.[28]
References
Notes
- ^ TM 9-1300-214. US Army.
- ^ Davis (1943) Volume II.
- ^ MacDonald and Mack Partnership (1984, p. 18)
- ^ Baxter III (1968, p. 27, 42, 255–259)
- ^ Simmons (1948), Part II and III.
- ^ John Sweetman, The Dambusters Raid (London: Cassell Military Paperbacks, 2002), p. 144).
- ^ Luo, K.-M., Lin, S.-H., Chang, J.-G., Huang, T.-H. (2002). "Evaluations of kinetic parameters and critical runaway conditions in the reaction system of hexamine-nitric acid to produce RDX in a non-isothermal batch reactor". Journal of Loss Prevention in the Process Industries. 15 (2): 119–127. doi:10.1016/S0950-4230(01)00027-4.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Urbanski, Tadeusz (1967), Chemistry and Technology of Explosives, vol. 3, PWN - Polish Scientific Publishers and Pergamon Press, pp. =77-119
{{citation}}: CS1 maint: extra punctuation (link) - ^ Urbanski (1967, p. 125) harvtxt error: multiple targets (2×): CITEREFUrbanski1967 (help) credits "G. C. V. Herz" for the patent, but the patentee is Edmund von Herz.
- ^ a b c von Herz (1921)
- ^ a b c von Herz (1922)
- ^ a b c Cocroft (2000, pp. 210–211)
- ^ Akhavan (2004)
- ^ a b Hornby (1958, pp. 112–114)
- ^ a b c Simmons, Forster & Bowden (1948) harvtxt error: multiple targets (2×): CITEREFSimmonsForsterBowden1948 (help)
- ^ a b c d e f g h i j k Baxter III (1968, pp. 253–239)
- ^ a b Gilman (1953, p. 985)
- ^ Schiessler & Ross (1947)
- ^ a b c Baxter III (1968, pp. 253–254)
- ^ MacDonald and Mack Partnership (1984, p. 19)
- ^ a b MacDonald and Mack Partnership (1984, p. 18)
- ^ These were not the only laboratories to work on RDX, Gilman's 1953 account of the Ross-Schiessler method was based on unpublished work from laboratories at the Universities of Michigan, Pennsylvania, Cornell, Harvard, Vanderbilt, McGill (Canada), Bristol (UK), Sheffield (UK), Pennsylvania State College and the UK's Research Department.
- ^ Bachmann & Sheehan (1949)
- ^ MacDonald and Mack Partnership (1984, p. 32)
- ^ U.S. Court of Appeals for the Ninth Circuit (February 2, 2010). "U.S. v. Ressam" (PDF). Retrieved February 27, 2010.
- ^ "Complaint; U.S. v. Ressam" (PDF). NEFA Foundation. December 1999. Retrieved February 26, 2010.
- ^ "Mumbai". The Times of India. Oct 2, 2006.
- ^ "Moscow Metro bombing masterminds 'will be destroyed'". BBC News. March 29, 2010. Retrieved April 2, 2010.
Bibliography
- Akhavan, Jacqueline (2004), The Chemistry of Explosives, Cambridge, UK: Royal Society of Chemistry, ISBN 0-85404-640-2
- Bachmann, W. E.; Sheehan, John C. (May 1949), "A New Method of Preparing the High Explosive RDX", Journal of the American Chemical Society, 71 (5): 1842–1845, doi:10.1021/ja01173a092
- Baxter III, James Phinney (1968) [1946], Scientists Against Time (MIT Paperback ed.), Cambridge, MA: MIT Press, ISBN 978-0262520126, OCLC 476611116
- Cocroft, Wayne D. (2000), Dangerous Energy: The archaeology of gunpowder and military explosives manufacture, Swindon: English Heritage, ISBN 1-85074-718-0
- Cooper, Paul W. (1996), Explosives Engineering, New York: Wiley-VCH, ISBN 0-471-18636-8
- Gilman, Henry (1953), "The Chemistry of Explosives", Organic Chemistry an Advanced Treatise, vol. III, Wiley; Chapman & Hall, pp. 985–986
- Davis, Tenney L. (1943), The Chemistry of Powder and Explosives, vol. II, New York: John Wiley & Sons Inc.
- Hale, George C. (November 3, 1925), "The Nitration of Hexamethylenetetramine", Journal of the American Chemical Society, 47 (11): 2754–2763, doi:10.1021/ja01688a017
-
{{citation}}: Empty citation (help) - Improvements relating to Explosives
{{citation}}: Cite has empty unknown parameter:|description=(help); Unknown parameter|country-code=ignored (help); Unknown parameter|inventor-first=ignored (help); Unknown parameter|inventor-last=ignored (help); Unknown parameter|issue-date=ignored (help); Unknown parameter|patent-number=ignored (help) - Explosive
{{citation}}: Cite has empty unknown parameter:|description=(help); Unknown parameter|country-code=ignored (help); Unknown parameter|inventor-first=ignored (help); Unknown parameter|inventor-last=ignored (help); Unknown parameter|issue-date=ignored (help); Unknown parameter|patent-number=ignored (help) - Hornby, William (1958), Factories and Plant, History of the Second World War: United Kingdom Civil Series, London: Her Majesty's Stationery Office; Longmans, Green and Co.
- MacDonald and Mack Partnership (August 1984), Final Properties Report: Newport Army Ammunition Plant (PDF), vol. AD-A175 818, National Park Service
- Meyer, Rudolf (1987), Explosives (3rd ed.), VCH Publishers, ISBN 0-89573-600-4
-
{{citation}}: Empty citation (help) - Simmons, W.H.; Forster, A.; Bowden, R. C. (August 1948), "The Manufacture of R.D.X. in Great Britain: Part II – Raw Materials and Ancillary Processes", The Industrial Chemist, 24: 530–545
{{citation}}: CS1 maint: date and year (link) - Simmons, W.H.; Forster, A.; Bowden, R. C. (September 1948), "The Manufacture of R.D.X. in Great Britain: Part III – Production of the Explosive", The Industrial Chemist, 24: 593–601
{{citation}}: CS1 maint: date and year (link) - Urbanski, Tadeusz (1967), Chemistry and Technology of Explosives, vol. III (First English ed.), Warszawa: PWN - Polish Scientific Publishers and Pergamon Press, OCLC 499857211. Translation by Marian Jurecki, edited by Sylvia Laverton. See also ISBN 9780080104010.
- Urbanski translation http://openlibrary.org/books/OL3160546M/Chemistry_and_technology_of_explosives, Macmillan, NY, 1964, ISBN 0080262066.
External links
- ADI Limited (Australia)
- NLM Hazardous Substances Databank (USA) – Cyclonite (RDX)
- GlobalSecurity.org, Explosives - Compositions, Alexandria, VA: GlobalSecurity.org, retrieved September 1, 2010
[] Error: {{Lang}}: no text (help)