N-Phenylacetyl-L-prolylglycine ethyl ester: Difference between revisions
Bon courage (talk | contribs) |
|||
| Line 54: | Line 54: | ||
==Legal status== |
==Legal status== |
||
*'''Hungary:''' As of 25 August 2020, Noopept is added to the controlled psychoactive substances list, prohibiting production, sale, import, storage and use.<ref>[https://magyarkozlony.hu/hivatalos-lapok/fsmDhb9y0CRKX2SkxRDw5f37b222dc2d1/dokumentumok/bc9ef7aef40a107a518ab17e1ac35adf2735e291/letoltes MAGYARORSZÁG HIVATALOS LAPJA]. Retrieved 2021-04-28.</ref> |
|||
*'''Russia:''' Noopept in Russia is a drug of medicine and is available without a prescription.<ref>{{cite web | title = Государственный реестр лекарственных средств | url = http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=6e1f901d-6924-4721-87d5-c82880be8286&t= }}</ref> |
*'''Russia:''' Noopept in Russia is a drug of medicine and is available without a prescription.<ref>{{cite web | title = Государственный реестр лекарственных средств | url = http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=6e1f901d-6924-4721-87d5-c82880be8286&t= }}</ref> |
||
*'''United Kingdom:''' Omberacetam is illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26, 2016.<ref>{{cite web | title = Psychoactive Substances Act 2016 | work = Legislation.gov.uk | url = http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted }}</ref> |
*'''United Kingdom:''' Omberacetam is illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26, 2016.<ref>{{cite web | title = Psychoactive Substances Act 2016 | work = Legislation.gov.uk | url = http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted }}</ref> |
||
*'''United States:''' The Food and Drug Administration has issued import alerts for imports of omberacetam, considering it an analog of piracetam.<ref>{{cite journal |last1=Cohen |first1=Pieter A. |last2=Zakharevich |first2=Igor |last3=Gerona |first3=Roy |title=Presence of Piracetam in Cognitive Enhancement Dietary Supplements |journal=JAMA Internal Medicine |date=1 March 2020 |volume=180 |issue=3 |pages=458–459 |doi=10.1001/jamainternmed.2019.5507|pmid=31764936 |pmc=6902196 }}</ref> FDA considers such racetam-family substances Active Pharmaceutical Ingredients (APIs) that require new drug applications and adequate labelling before being imported.<ref>{{cite web | url = https://www.accessdata.fda.gov/cms_ia/importalert_202.html | title = Import alert 66-66 }}</ref> Similarly, warnings have been issued for claims of medical and pharmacological effects of this officially non-drug substance.<ref>{{cite web | url = https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/peak-nootropics-llc-aka-advanced-nootropics-557887-02052019 | title = FDA Warning letter: Peak Nootropics LLC aka Advanced Nootropics }}</ref> Despite these FDA enforcement actions, omberacetam is sold in over-the-counter supplements in this US with some products formulated with dosages greater than pharmaceutical levels.<ref>{{cite journal |last1=Cohen |first1=Pieter A. |last2=Avula |first2=Bharathi |last3=Wang |first3=Yan Hong |last4=Zakharevich |first4=Igor |last5=Khan |first5=Ikhlas |title=Five unapproved drugs found in cognitive enhancement supplements |journal=Neurology: Clinical Practice |date=23 September 2020 |pages=10.1212/CPJ.0000000000000960 |doi=10.1212/CPJ.0000000000000960}}</ref> |
*'''United States:''' The Food and Drug Administration has issued import alerts for imports of omberacetam, considering it an analog of piracetam.<ref>{{cite journal |last1=Cohen |first1=Pieter A. |last2=Zakharevich |first2=Igor |last3=Gerona |first3=Roy |title=Presence of Piracetam in Cognitive Enhancement Dietary Supplements |journal=JAMA Internal Medicine |date=1 March 2020 |volume=180 |issue=3 |pages=458–459 |doi=10.1001/jamainternmed.2019.5507|pmid=31764936 |pmc=6902196 }}</ref> FDA considers such racetam-family substances Active Pharmaceutical Ingredients (APIs) that require new drug applications and adequate labelling before being imported.<ref>{{cite web | url = https://www.accessdata.fda.gov/cms_ia/importalert_202.html | title = Import alert 66-66 }}</ref> Similarly, warnings have been issued for claims of medical and pharmacological effects of this officially non-drug substance.<ref>{{cite web | url = https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/peak-nootropics-llc-aka-advanced-nootropics-557887-02052019 | title = FDA Warning letter: Peak Nootropics LLC aka Advanced Nootropics }}</ref> Despite these FDA enforcement actions, omberacetam is sold in over-the-counter supplements in this US with some products formulated with dosages greater than pharmaceutical levels.<ref>{{cite journal |last1=Cohen |first1=Pieter A. |last2=Avula |first2=Bharathi |last3=Wang |first3=Yan Hong |last4=Zakharevich |first4=Igor |last5=Khan |first5=Ikhlas |title=Five unapproved drugs found in cognitive enhancement supplements |journal=Neurology: Clinical Practice |date=23 September 2020 |pages=10.1212/CPJ.0000000000000960 |doi=10.1212/CPJ.0000000000000960}}</ref> |
||
== See also == |
== See also == |
||
Revision as of 16:16, 28 April 2021
This article needs more reliable medical references for verification or relies too heavily on primary sources. (January 2021) |  |
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Noopept |
| Other names | N-Phenylacetyl-l-prolylglycine ethyl ester; GVS-111 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
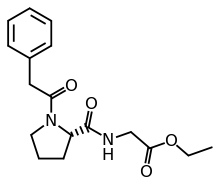
| Formula | C17H22N2O4 |
| Molar mass | 318.373 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
-Phenylacetyl-l-prolylglycine ethyl ester is promoted as a nootropic and is a prodrug of cycloprolylglycine.[1] Other names include the brand name Noopept (Russian: Ноопепт), developmental code GVS-111; proposed INN omberacetam.[1][2][3]
Its synthesis was first reported in 1996.[1] It is orally available, and as of 2017 its metabolism and elimination half-life were not well understood, as cycloprolylglycine had not been measured in humans following administration.[1]
As of 2018 there have been many studies conducted in cells and in animal models, and there has been one clinical trial with 41 subjects comparing Noopept and piracetam in people with traumatic brain injury.[1]
Pharmacology
All theories regarding Noopept's mechanism of action are based on observations from animal studies. One oft-cited study (originally published in Russian) conducted on rats, suggests that Noopept works via the "antioxidant effect, the antiinflammatory action, and the ability to inhibit the neurotoxicity of excess calcium and glutamate, and to improve the blood rheology".[4] To date, no trial has been conducted on humans to confirm these theories.
Dosage
Noopept is frequently dosed at 10-30mg per day. However, there is no solid evidence indicating that any dose of Noopept is optimal. Few human trials have ever been carried out on Noopept, and as one meta-analysis notes, animal studies have used doses ranging from 0.1mg/kg bodyweight to 10mg/kg bodyweight.[5] Furthermore, no long-term studies have been done to evaluate the lasting effects of chronic use at any given dose; the longest human study lasted for 56 days.[6] There is, therefore, no dose of Noopept which may be called "safe".
Legal status
- Hungary: As of 25 August 2020, Noopept is added to the controlled psychoactive substances list, prohibiting production, sale, import, storage and use.[7]
- Russia: Noopept in Russia is a drug of medicine and is available without a prescription.[8]
- United Kingdom: Omberacetam is illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26, 2016.[9]
- United States: The Food and Drug Administration has issued import alerts for imports of omberacetam, considering it an analog of piracetam.[10] FDA considers such racetam-family substances Active Pharmaceutical Ingredients (APIs) that require new drug applications and adequate labelling before being imported.[11] Similarly, warnings have been issued for claims of medical and pharmacological effects of this officially non-drug substance.[12] Despite these FDA enforcement actions, omberacetam is sold in over-the-counter supplements in this US with some products formulated with dosages greater than pharmaceutical levels.[13]
See also
External links
References
- ^ a b c d e "Noopept Information". Examine.com. Retrieved 6 April 2017.
- ^ "Proposed INN List 117" (PDF). WHO Drug Information. 31 (2): 308. 2017.
- ^ "GVS 111". AdisInsight. Retrieved 12 May 2018.
- ^ Ostrovskaia RU, Gudasheva TA, Voronina TA, Seredenin SB (September 2002). "[The original novel nootropic and neuroprotective agent noopept]". Eksperimental'naia i Klinicheskaia Farmakologiia. 65 (5): 66–72. PMID 12596521.
- ^ Tardner, P (2020). "Finding the optimal dosage fornootropic agent Noopept: An analysis of available literature" (PDF). International Journal of Environmental Science and Technology.
- ^ Neznamov, G. G.; Teleshova, E. S. (2009). "Comparative studies of Noopept and piracetam in the treatment of patients with mild cognitive disorders in organic brain diseases of vascular and traumatic origin". Neuroscience and Behavioral Physiology. 39 (3): 311–321. doi:10.1007/s11055-009-9128-4. ISSN 0097-0549. PMID 19234797.
- ^ MAGYARORSZÁG HIVATALOS LAPJA. Retrieved 2021-04-28.
- ^ "Государственный реестр лекарственных средств".
- ^ "Psychoactive Substances Act 2016". Legislation.gov.uk.
- ^ Cohen, Pieter A.; Zakharevich, Igor; Gerona, Roy (1 March 2020). "Presence of Piracetam in Cognitive Enhancement Dietary Supplements". JAMA Internal Medicine. 180 (3): 458–459. doi:10.1001/jamainternmed.2019.5507. PMC 6902196. PMID 31764936.
- ^ "Import alert 66-66".
- ^ "FDA Warning letter: Peak Nootropics LLC aka Advanced Nootropics".
- ^ Cohen, Pieter A.; Avula, Bharathi; Wang, Yan Hong; Zakharevich, Igor; Khan, Ikhlas (23 September 2020). "Five unapproved drugs found in cognitive enhancement supplements". Neurology: Clinical Practice: 10.1212/CPJ.0000000000000960. doi:10.1212/CPJ.0000000000000960.
