Intraepithelial lymphocyte: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
'''Intraepithelial lymphocytes''' (IEL) are [[lymphocyte]]s found in the [[epithelium|epithelial]] layer of mammalian [[Mucous membrane|mucosal linings]], such as the [[gastrointestinal tract|gastrointestinal (GI) tract]] and [[reproductive tract]].<ref name="pmid16787773" /> Epithelium of small intestine contains approximately 1 IEL |
'''Intraepithelial lymphocytes''' (IEL) are [[lymphocyte]]s found in the [[epithelium|epithelial]] layer of mammalian [[Mucous membrane|mucosal linings]], such as the [[gastrointestinal tract|gastrointestinal (GI) tract]] and [[reproductive tract]].<ref name="pmid16787773" /> Epithelium of small intestine contains approximately 1 IEL per 10 enterocytes <ref name=":6">{{Cite journal|last=Olivares-Villagómez|first=Danyvid|last2=Van Kaer|first2=Luc|date=2018-04|title=Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier|url=https://linkinghub.elsevier.com/retrieve/pii/S1471490617302132|journal=Trends in Immunology|language=en|volume=39|issue=4|pages=264–275|doi=10.1016/j.it.2017.11.003|pmc=PMC8056148|pmid=29221933}}</ref>. However, unlike other [[T cell]]s, IELs do not need priming. Upon encountering antigens, they immediately release [[cytokines]] and cause killing of infected target cells. In the GI tract, they are components of [[gut-associated lymphoid tissue]] (GALT).<ref>Defranco, Anthony L; Locksley, Richard M; Robertson, Miranda. 2007. Immunity: The Immune Response in Infection and Inflammatory Disease. New Science Press Ltd. 218-219.</ref> |
||
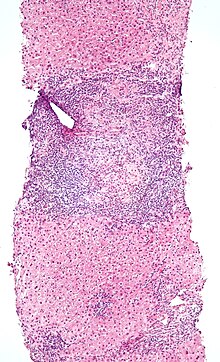
Based on expression of either an αβ T-cell receptor (TCR) or a γδ TCR IEL T cells can be divided into two major groups. In mice both groups are retained in almost equal proportions.<ref name=":3">{{cite journal | vauthors = Sheridan BS, Lefrançois L | title = Intraepithelial lymphocytes: to serve and protect | journal = Current Gastroenterology Reports | volume = 12 | issue = 6 | pages = 513–21 | date = December 2010 | pmid = 20890736 | pmc = 3224371 | doi = 10.1007/s11894-010-0148-6 }}</ref> In humans, the majority of IELs are alpha beta T cells. 15% of IELs are gamma delta T cells and thus represent a minor component of human IELs. However, IELs significantly increase under certain conditions, such as [[Coeliac disease|celiac disease]].<ref name="pmid16787773" />[[File:Primary biliary cirrhosis low mag.jpg|thumb|primary biliary cirrhosis. Bile duct intraepithelial lymphocytes]] |
Based on expression of either an αβ T-cell receptor (TCR) or a γδ TCR IEL T cells can be divided into two major groups. In mice both groups are retained in almost equal proportions.<ref name=":3">{{cite journal | vauthors = Sheridan BS, Lefrançois L | title = Intraepithelial lymphocytes: to serve and protect | journal = Current Gastroenterology Reports | volume = 12 | issue = 6 | pages = 513–21 | date = December 2010 | pmid = 20890736 | pmc = 3224371 | doi = 10.1007/s11894-010-0148-6 }}</ref> In humans, the majority of IELs are alpha beta T cells. 15% of IELs are gamma delta T cells and thus represent a minor component of human IELs. However, IELs significantly increase under certain conditions, such as [[Coeliac disease|celiac disease]].<ref name="pmid16787773" />[[File:Primary biliary cirrhosis low mag.jpg|thumb|primary biliary cirrhosis. Bile duct intraepithelial lymphocytes]] |
||
| Line 26: | Line 26: | ||
Alternatively, elevated IEL populations can be a marker for developing neoplasia in the tissue such as found in [[cervical cancer|cervical]] and [[prostate cancer|prostate]] cancers, as well as some [[colon cancer|colorectal cancers]], particularly those associated with [[Lynch syndrome]] (hereditary non-polyposis colon cancer <nowiki><HNPCC></nowiki>).<ref name=bellizzi>{{cite journal | vauthors = Bellizzi AM, Frankel WL | title = Colorectal cancer due to deficiency in DNA mismatch repair function: a review | journal = Advances in Anatomic Pathology | volume = 16 | issue = 6 | pages = 405–17 | date = November 2009 | pmid = 19851131 | doi = 10.1097/PAP.0b013e3181bb6bdc }}</ref> IELs themselves can, when chronically activated, undergo mutation that can lead to [[lymphoma]].<ref>{{cite journal | vauthors = Meresse B, Malamut G, Cerf-Bensussan N | title = Celiac disease: an immunological jigsaw | journal = Immunity | volume = 36 | issue = 6 | pages = 907–19 | date = June 2012 | pmid = 22749351 | doi = 10.1016/j.immuni.2012.06.006 | doi-access = free }}</ref> |
Alternatively, elevated IEL populations can be a marker for developing neoplasia in the tissue such as found in [[cervical cancer|cervical]] and [[prostate cancer|prostate]] cancers, as well as some [[colon cancer|colorectal cancers]], particularly those associated with [[Lynch syndrome]] (hereditary non-polyposis colon cancer <nowiki><HNPCC></nowiki>).<ref name=bellizzi>{{cite journal | vauthors = Bellizzi AM, Frankel WL | title = Colorectal cancer due to deficiency in DNA mismatch repair function: a review | journal = Advances in Anatomic Pathology | volume = 16 | issue = 6 | pages = 405–17 | date = November 2009 | pmid = 19851131 | doi = 10.1097/PAP.0b013e3181bb6bdc }}</ref> IELs themselves can, when chronically activated, undergo mutation that can lead to [[lymphoma]].<ref>{{cite journal | vauthors = Meresse B, Malamut G, Cerf-Bensussan N | title = Celiac disease: an immunological jigsaw | journal = Immunity | volume = 36 | issue = 6 | pages = 907–19 | date = June 2012 | pmid = 22749351 | doi = 10.1016/j.immuni.2012.06.006 | doi-access = free }}</ref> |
||
== Classification == |
|||
IELs can be divided to different subpopulations based on molecular markers and thorough classification to several subpopulations is done by the presence of TCR, CD8αα and by origin. |
|||
=== Induced TCR<sup>+</sup> IELs === |
|||
Populations of induced IELs emerge from conventional peripheral CD4<sup>+</sup> T-cells. They have in common the expression of TCR and can also express CD8αα that is induced after migration into the intestinal epithelium. |
|||
==== TCRαβ<sup>+</sup>CD4<sup>+</sup> IELs ==== |
|||
TCRαβ<sup>+</sup>CD4<sup>+</sup> IELs arise from conventional peripheral CD4<sup>+</sup> T-cells. These cells migrate into the intestinal epithelium as effector or tissue-resident memory T cells. |
|||
In mice, up to 50 % of these IELs can express CD8αα homodimer, which they acquire in the intestinal epithelium after external stimuli such as TGF-β, IFN-γ, IL-27 and retinoic acid. Function of TCRαβ<sup>+</sup> CD4<sup>+</sup> CD8αα<sup>+</sup> IELs is unclear. Even though they express granzymes and have cytolytic properties, it has been suggested that they can have also regulatory properties in the context of chronic intestinal inflammation <ref name=":6" /><ref name=":7">{{Cite journal|last=Ma|first=Haitao|last2=Qiu|first2=Yuan|last3=Yang|first3=Hua|date=2021-02|title=Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity|url=https://onlinelibrary.wiley.com/doi/10.1002/JLB.3RU0220-111|journal=Journal of Leukocyte Biology|language=en|volume=109|issue=2|pages=339–347|doi=10.1002/JLB.3RU0220-111|issn=0741-5400|pmc=PMC7891415|pmid=32678936}}</ref>. |
|||
==== TCRαβ<sup>+</sup>CD8αβ<sup>+</sup> IELs ==== |
|||
These IELs also emerge from peripherally activated conventional T-cells but from CD8<sup>+</sup> population and they home to the intestinal epithelium, where they function as effector or memory cells. They continuously express integrin β7, granzyme B, CD103 and CD69 and produce lower amounts of TNF-α and IFN-γ as opposed to conventional CD8<sup>+</sup> T-cells <ref name=":8">{{Cite journal|last=McDonald|first=Benjamin D.|last2=Jabri|first2=Bana|last3=Bendelac|first3=Albert|date=2018-08|title=Diverse developmental pathways of intestinal intraepithelial lymphocytes|url=http://www.nature.com/articles/s41577-018-0013-7|journal=Nature Reviews Immunology|language=en|volume=18|issue=8|pages=514–525|doi=10.1038/s41577-018-0013-7|issn=1474-1733|pmc=PMC6063796|pmid=29717233}}</ref>. |
|||
Some of these cells also express CD8αα homodimer and these cells can be pathogenic during coeliac disease in humans <ref name=":6" />. |
|||
=== Natural TCR<sup>+</sup> IELs === |
|||
Natural IELs also express TCR and home to the epithelium right after development, where they acquire expression of CD8αα. |
|||
==== TCRαβ<sup>+</sup> IELs ==== |
|||
In mice, these IELs are the most abundant at birth and with age their numbers decrease. In humans, these cells are present during gestation but are very rare in adulthood. TCRαβ<sup>+</sup> IELs develop in thymus where they undergo agonist positive selection and thereby are self-reactive. Nevertheless, they have regulatory properties and protect against colitis in animal experiments. These cells are influenced by normal intestinal microbiota and vitamin D. NOD2 receptor expressed by antigen presenting cells and epithelial cells in the intestine recognizes microbes and triggers production of IL-15 cytokine, which promotes TCRαβ<sup>+</sup>CD8αα<sup>+</sup> IELs <ref name=":6" />. |
|||
==== TCRγδ<sup>+</sup> IELs ==== |
|||
TCRγδ<sup>+</sup> IELs develop outside of thymus and their maintenance and function in the intestinal epithelium is influenced by cross-talk with enterocytes. Moreover, they can migrate through the epithelium with the help of interactions with epithelial cells <ref>{{Cite journal|last=Hoytema van Konijnenburg|first=David P.|last2=Reis|first2=Bernardo S.|last3=Pedicord|first3=Virginia A.|last4=Farache|first4=Julia|last5=Victora|first5=Gabriel D.|last6=Mucida|first6=Daniel|date=2017-11|title=Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection|url=https://linkinghub.elsevier.com/retrieve/pii/S009286741731005X|journal=Cell|language=en|volume=171|issue=4|pages=783–794.e13|doi=10.1016/j.cell.2017.08.046|pmc=PMC5670000|pmid=28942917}}</ref>. These cells have cytotoxic properties and produce cytokines TGF-β, TNF-α, IFN-γ, IL-13 and IL-10 and antimicrobial peptides. In mice, most of these cells express Vγ7 and on the other hand, in humans, most are Vγ4 positive. Their function resides in protection of the intestinal barrier against pathogens early in the infection and later they quench the inflammation and protect the barrier from tissue damage. Similar facts have been found in the context of colitis. These cells seem to have pathogenic role at the beginning, whereas later they protect the epithelium against tissue damage <ref name=":7" />. |
|||
=== TCR<sup>-</sup> IELs === |
|||
IELs that do not express TCR. |
|||
==== ILC-like IELs ==== |
|||
These cells show properties of ILC1 cells, more precisely properties of NK cells. In humans, they are elevated during Crohn´s disease and in mice, they are pathogenic during colitis <ref name=":8" />. |
|||
==== iCD8α ==== |
|||
These innate lymphocytes express homodimer CD8αα and CD3 and develop outside of thymus. They have cytotoxic and phagocytic properties, express MHC II and thereby can present antigens to conventional CD4<sup>+</sup> T-cells. iCD8α protect against bacterial infections and promotes experimental colitis <ref name=":6" />. |
|||
==== TCR<sup>-</sup>iCD3<sup>+</sup>CD8αα<sup>-</sup> IELs ==== |
|||
These cells are very similar to iCD8α population and it is unclear if this is a different subset of cells or only precursors of iCD8α <ref name=":6" />. |
|||
== See also == |
== See also == |
||
Revision as of 14:47, 30 June 2021
Intraepithelial lymphocytes (IEL) are lymphocytes found in the epithelial layer of mammalian mucosal linings, such as the gastrointestinal (GI) tract and reproductive tract.[1] Epithelium of small intestine contains approximately 1 IEL per 10 enterocytes [2]. However, unlike other T cells, IELs do not need priming. Upon encountering antigens, they immediately release cytokines and cause killing of infected target cells. In the GI tract, they are components of gut-associated lymphoid tissue (GALT).[3]
Based on expression of either an αβ T-cell receptor (TCR) or a γδ TCR IEL T cells can be divided into two major groups. In mice both groups are retained in almost equal proportions.[4] In humans, the majority of IELs are alpha beta T cells. 15% of IELs are gamma delta T cells and thus represent a minor component of human IELs. However, IELs significantly increase under certain conditions, such as celiac disease.[1]
Phenotype
The majority of IELs (80%) are CD3+, and over 75% of these also express CD8. IELs can be divided into two major subsets based on their CD8 coreceptor expression.[4] One subset of IELs typically express activation marker CD8αα and some IELs express CD8αβ+ marker (CD8αβ promotes TCR activation, whereas CD8αα suppresses TCR signals).
In both humans and mice IELs express higher levels of CD103, activation marker CD69, granzyme B and perforin cytolytic granules. CD25 expression is lower in comparison with effector memory T cells.[5][6]
CD8αα
Expression of CD8αα is an important phenotypic feature of IELs, but not all IELs subpopulations express this molecule. CD8αα homodimer is an alternative isoform to classical CD8αβ heterodimer, which is expressed on conventional CD8 T-cells. CD8αα is mainly expressed by effector or mature antigen-experienced cells in the gut. This molecule can bind MHC I, but, opposed to the function of CD8αβ, CD8αα reduces sensitivity of TCR towards antigens. Thus, when recognizing MHC I, CD8αα functions as a repressor of activation [7].
CD8αα can also recognize thymus leukemia (TL) antigen, which is a non-classical MHC I molecule that is expressed in thymus and in intestinal epithelium. Interaction between TL and CD8αα does not serve for migration of IELs into the epithelium, but it is important for modulating immune response of IELs [8]. It has been suggested that cross-talk between TL and CD8αα might regulate IELs survival and proliferation [7]. More accurately, TL prevents proliferation of IELs, when there is co-occurrence of weak TCR stimulation [8].
Development
Induced IELs (TCRαβ+ CD8αβ+) are generated from naive T cells during an immune response. TCRαβ+ CD8αα (natural IELs) cells differentiate in the thymus.[5][9]
Development and cytolytic activation are independent of live micro-organisms but they become cytolytic in response to the exogenous antigenic substances other than live micro-organisms in the gut. IEL T cells acquire their activated memory phenotype post-thymically, in response to antigens encountered in the periphery.[10]
Function
Their role in immune system is crucial because IELs provide a first line of defense at this extensive barrier with the outside world. All IEL T cells are antigen-experienced T cells, which typically display a cytotoxic functional phenotype. IELs mediate antigen-specific delayed-type hypersensitivity (DTH) responses, exhibit virus-specific CTL function, to express natural killer (NK)-like activity and produce a local graft-versus-host reaction (GVHR) when transferred to semiallogeneic hosts. IELs are also able to produce a variety of cytokines which are characteristically produced by Th1- and Th2-type cells and can also provide help for B cell responses.[5][9][10]
Pathology
An elevated IEL population, as determined by biopsy, typically indicates ongoing inflammation within the mucosa. In diseases such as celiac sprue, IEL elevation throughout the small intestine is one of many specific markers.[1] IELs have heightened activated status that can lead to inflammatory disease such as IBD, promote cancer development and progression,[11] or become the malignant cells in enteropathy-associated T-cell lymphoma, a lymphoma that is a complication of celiac sprue.[12][13]
Alternatively, elevated IEL populations can be a marker for developing neoplasia in the tissue such as found in cervical and prostate cancers, as well as some colorectal cancers, particularly those associated with Lynch syndrome (hereditary non-polyposis colon cancer <HNPCC>).[14] IELs themselves can, when chronically activated, undergo mutation that can lead to lymphoma.[15]
Classification
IELs can be divided to different subpopulations based on molecular markers and thorough classification to several subpopulations is done by the presence of TCR, CD8αα and by origin.
Induced TCR+ IELs
Populations of induced IELs emerge from conventional peripheral CD4+ T-cells. They have in common the expression of TCR and can also express CD8αα that is induced after migration into the intestinal epithelium.
TCRαβ+CD4+ IELs
TCRαβ+CD4+ IELs arise from conventional peripheral CD4+ T-cells. These cells migrate into the intestinal epithelium as effector or tissue-resident memory T cells.
In mice, up to 50 % of these IELs can express CD8αα homodimer, which they acquire in the intestinal epithelium after external stimuli such as TGF-β, IFN-γ, IL-27 and retinoic acid. Function of TCRαβ+ CD4+ CD8αα+ IELs is unclear. Even though they express granzymes and have cytolytic properties, it has been suggested that they can have also regulatory properties in the context of chronic intestinal inflammation [2][16].
TCRαβ+CD8αβ+ IELs
These IELs also emerge from peripherally activated conventional T-cells but from CD8+ population and they home to the intestinal epithelium, where they function as effector or memory cells. They continuously express integrin β7, granzyme B, CD103 and CD69 and produce lower amounts of TNF-α and IFN-γ as opposed to conventional CD8+ T-cells [17].
Some of these cells also express CD8αα homodimer and these cells can be pathogenic during coeliac disease in humans [2].
Natural TCR+ IELs
Natural IELs also express TCR and home to the epithelium right after development, where they acquire expression of CD8αα.
TCRαβ+ IELs
In mice, these IELs are the most abundant at birth and with age their numbers decrease. In humans, these cells are present during gestation but are very rare in adulthood. TCRαβ+ IELs develop in thymus where they undergo agonist positive selection and thereby are self-reactive. Nevertheless, they have regulatory properties and protect against colitis in animal experiments. These cells are influenced by normal intestinal microbiota and vitamin D. NOD2 receptor expressed by antigen presenting cells and epithelial cells in the intestine recognizes microbes and triggers production of IL-15 cytokine, which promotes TCRαβ+CD8αα+ IELs [2].
TCRγδ+ IELs
TCRγδ+ IELs develop outside of thymus and their maintenance and function in the intestinal epithelium is influenced by cross-talk with enterocytes. Moreover, they can migrate through the epithelium with the help of interactions with epithelial cells [18]. These cells have cytotoxic properties and produce cytokines TGF-β, TNF-α, IFN-γ, IL-13 and IL-10 and antimicrobial peptides. In mice, most of these cells express Vγ7 and on the other hand, in humans, most are Vγ4 positive. Their function resides in protection of the intestinal barrier against pathogens early in the infection and later they quench the inflammation and protect the barrier from tissue damage. Similar facts have been found in the context of colitis. These cells seem to have pathogenic role at the beginning, whereas later they protect the epithelium against tissue damage [16].
TCR- IELs
IELs that do not express TCR.
ILC-like IELs
These cells show properties of ILC1 cells, more precisely properties of NK cells. In humans, they are elevated during Crohn´s disease and in mice, they are pathogenic during colitis [17].
iCD8α
These innate lymphocytes express homodimer CD8αα and CD3 and develop outside of thymus. They have cytotoxic and phagocytic properties, express MHC II and thereby can present antigens to conventional CD4+ T-cells. iCD8α protect against bacterial infections and promotes experimental colitis [2].
TCR-iCD3+CD8αα- IELs
These cells are very similar to iCD8α population and it is unclear if this is a different subset of cells or only precursors of iCD8α [2].
See also
References
- ^ a b c Hopper AD, Hurlstone DP, Leeds JS, McAlindon ME, Dube AK, Stephenson TJ, Sanders DS (November 2006). "The occurrence of terminal ileal histological abnormalities in patients with coeliac disease". Digestive and Liver Disease. 38 (11): 815–9. doi:10.1016/j.dld.2006.04.003. PMID 16787773.
- ^ a b c d e f Olivares-Villagómez, Danyvid; Van Kaer, Luc (2018-04). "Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier". Trends in Immunology. 39 (4): 264–275. doi:10.1016/j.it.2017.11.003. PMC 8056148. PMID 29221933.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Defranco, Anthony L; Locksley, Richard M; Robertson, Miranda. 2007. Immunity: The Immune Response in Infection and Inflammatory Disease. New Science Press Ltd. 218-219.
- ^ a b Sheridan BS, Lefrançois L (December 2010). "Intraepithelial lymphocytes: to serve and protect". Current Gastroenterology Reports. 12 (6): 513–21. doi:10.1007/s11894-010-0148-6. PMC 3224371. PMID 20890736.
- ^ a b c Mayassi T, Jabri B (September 2018). "Human intraepithelial lymphocytes". Mucosal Immunology. 11 (5): 1281–1289. doi:10.1038/s41385-018-0016-5. PMC 6178824. PMID 29674648.
- ^ Lambolez F, Mayans S, Cheroutre H (2013). Lymphocytes: Intraepithelial. American Cancer Society. doi:10.1002/9780470015902.a0001197.pub3. ISBN 9780470015902.
{{cite book}}:|work=ignored (help) - ^ a b Cheroutre, Hilde; Lambolez, Florence (2008-02). "Doubting the TCR Coreceptor Function of CD8αα". Immunity. 28 (2): 149–159. doi:10.1016/j.immuni.2008.01.005.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Olivares-Villagómez, Danyvid; Van Kaer, Luc (2010-11). "TL and CD8αα: Enigmatic partners in mucosal immunity". Immunology Letters. 134 (1): 1–6. doi:10.1016/j.imlet.2010.09.004. PMC 2967663. PMID 20850477.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ a b Sim GK (1995-01-01). "Intraepithelial lymphocytes and the immune system". Advances in Immunology. 58: 297–343. doi:10.1016/s0065-2776(08)60622-7. ISBN 9780120224586. PMID 7741030.
- ^ a b McGhee, Jerry R. (1998-01-01). "Mucosa-Associated Lymphoid Tissue (MALT)". In Delves, Peter J. (ed.). Encyclopedia of Immunology (Second ed.). Elsevier. pp. 1774–1780. doi:10.1006/rwei.1999.0448. ISBN 9780122267659.
- ^ Cheroutre H, Lefrancois L (2015-01-01). "Chapter 35 - Intraepithelial TCRαβ T Cells in Health and Disease". In Mestecky J, Strober W, Russell MW, Kelsall BL (eds.). Mucosal Immunology (Fourth ed.). Academic Press. pp. 733–748. doi:10.1016/b978-0-12-415847-4.00035-5. ISBN 9780124158474.
- ^ Ondrejka S, Jagadeesh D (December 2016). "Enteropathy-Associated T-Cell Lymphoma". Current Hematologic Malignancy Reports. 11 (6): 504–513. doi:10.1007/s11899-016-0357-7. PMID 27900603.
- ^ Chander U, Leeman-Neill RJ, Bhagat G (August 2018). "Pathogenesis of Enteropathy-Associated T Cell Lymphoma". Current Hematologic Malignancy Reports. 13 (4): 308–317. doi:10.1007/s11899-018-0459-5. PMID 29943210.
- ^ Bellizzi AM, Frankel WL (November 2009). "Colorectal cancer due to deficiency in DNA mismatch repair function: a review". Advances in Anatomic Pathology. 16 (6): 405–17. doi:10.1097/PAP.0b013e3181bb6bdc. PMID 19851131.
- ^ Meresse B, Malamut G, Cerf-Bensussan N (June 2012). "Celiac disease: an immunological jigsaw". Immunity. 36 (6): 907–19. doi:10.1016/j.immuni.2012.06.006. PMID 22749351.
- ^ a b Ma, Haitao; Qiu, Yuan; Yang, Hua (2021-02). "Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity". Journal of Leukocyte Biology. 109 (2): 339–347. doi:10.1002/JLB.3RU0220-111. ISSN 0741-5400. PMC 7891415. PMID 32678936.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ a b McDonald, Benjamin D.; Jabri, Bana; Bendelac, Albert (2018-08). "Diverse developmental pathways of intestinal intraepithelial lymphocytes". Nature Reviews Immunology. 18 (8): 514–525. doi:10.1038/s41577-018-0013-7. ISSN 1474-1733. PMC 6063796. PMID 29717233.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Hoytema van Konijnenburg, David P.; Reis, Bernardo S.; Pedicord, Virginia A.; Farache, Julia; Victora, Gabriel D.; Mucida, Daniel (2017-11). "Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection". Cell. 171 (4): 783–794.e13. doi:10.1016/j.cell.2017.08.046. PMC 5670000. PMID 28942917.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link)
