Talk:Cellulose: Difference between revisions
| Line 123: | Line 123: | ||
I was wondering why the phrase |
I was wondering why the phrase |
||
Plastics are typically polymers of high molecular mass, and may contain ... |
Plastics are typically polymers of high molecular mass, and may contain ... |
||
contains the word typically. I am not aware of any plastic that is not also a polymer. Comments ? |
contains the word typically, as if there are plastics that are not polymers. I am not aware of any plastic that is not also a polymer. Comments ? [[User:Sbo|Stefano Borini]] ([[User talk:Sbo|talk]]) 13:06, 29 July 2010 (UTC) |
||
Revision as of 13:07, 29 July 2010
| Chemicals: Core B‑class High‑importance | |||||||||||||
| |||||||||||||
Details on cellulose degradation needed
Cellulose recycling is one of the important bio-geo-cycling events that maintain the integrity of our ecosystem. There is no good write up on this. Also there is no material on its biochemistry. I have added a few material in the chemistry section, but require help in cleanup and material addition Nattu 10:53, 11 July 2006 (UTC)
the melting point is incorrect! decomp occurs before the boiling point not before the melting point.
please see http://sciencelab.com/msds.php?msdsId=9927490 —Preceding unsigned comment added by 141.219.34.107 (talk) 21:58, 21 June 2010 (UTC)
Cellulose Estimation
Cellulose can be extracted from biomass, but how do we estimate cellulose. What is cellulose estimation method? How is it done?
- A standard method using acetic acid and nitric acid, and anthrone dissolved in sulfuric acid was described in Updegraff DM (1969) Semimicro determination of cellulose in biological materials. Anal Biochem 32: 420-424
- I'll add this info to the article. --Slashme 06:50, 20 December 2005 (UTC)
biogeneration of cellulose
is there any place on wikipedia which describes how plants make cellulose?
Incorrect diagram
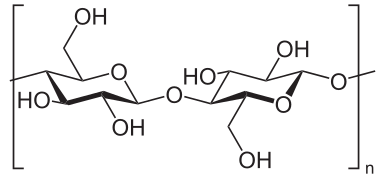
can someone fix the diagram of cellulose? the structure is incorrect, there needs to be an O atom either at C1 or C4 inside the brackets. Xcomradex 00:40, 12 June 2006 (UTC)
- I see the current diagram was incorrect as well, it now had two Oxygens in the repeating stucture! I have made an SVG of the diagram, and corrected the error. --Slashme 15:31, 17 October 2007 (UTC)
structures of cellulose
In literature the natural crystaline cellulose is usually referred to as Cellulose I (I alpha & I beta) and as Cellulose II after mercerization. Not sure how old the alpha, beta, gamma nomenclature from the article is, but I'd put the one I wrote in the article. JSchoeck; 14:41 (MET), 22.6.06
Fungi
Why is there no mention of cellulose being located in the cell walls of some fungi. D-rew 19:41, 9 September 2006 (UTC)
Cellulose production
Could someone please get an accurate figure on total cellulose production? 1.5 trillion tons is roughly how much could be produced annually if every square inch of land area on Earth were used solely for cellulose production 365 days a year. 72.224.97.185 03:37, 26 December 2006 (UTC)
Breakdown
What is produced when the substance is broken down. In more detail then the current description. Is glucose a product? —The preceding unsigned comment was added by 124.185.130.29 (talk) 08:40, 22 April 2007 (UTC).
Question
What bacteria digests cellulose, found in the gut of herbivores ? Is it cellumonas, cellulomonas or something completely different. Please reply fast. —Preceding unsigned comment added by 59.183.179.142 (talk) 06:43, August 26, 2007 (UTC)
- Fibrobacter succinogenes seems to be one of them, according to "Digestibility of cotton lint fiber and whole oilseeds by ruminal microorganisms", D.L. Palmquist, Animal Feed Science Technology 56 (1995) 23l-242. --Slashme 06:04, 17 October 2007 (UTC)
- Ruminococcus albus, Butyrivibrio fibrisolvens and Phanerochaete chrysosporium also seem to be relevant, but you'll have to chase the refs yourself, sorry!. --Slashme 06:10, 17 October 2007 (UTC)
Here's a quote from "Production of Ruminococcus flavefaciens growth inhibitor(s) by Ruminococcus albus, W.W. Chan, B.A. Dehority, Animal Feed Science and Technology 77 (1999) 61-71":
(Ruminococcus albus, a fibre-degrader; Butyrivibrio fibriosolvens CF3 and Clostridium proteoclasticum, xylan and protein degraders; and C. aminophilum, C. proteoclasticum, and Peptostreptococcus anaerobius, ammonia hyper-producing bacteria)
--Slashme 06:17, 17 October 2007 (UTC)
See also methanogen. --Slashme 15:33, 17 October 2007 (UTC)
Ive added notes regarding research published 20 Mar 2007 by Tokuda and Watanabe, they make a strong case that the termites they studied produce their own cellulase enzymes. R.F. Richholt (talk) 02:51, 22 April 2010 (UTC)
Which organic compound?
what kind of organic compound is cellulose a carbohydrate, lipid, protein, or is it a nucleic acid? PLEASE REPLY!!!! --72.28.207.37 21:08, 16 October 2007 (UTC)miki_dee
- It's a carbohydrate, but it cannot be digested by humans. --Slashme 05:38, 17 October 2007 (UTC)
- By the way, the second sentence in the article is "It is a structural polysaccharide derived from beta-glucose. If you don't know what a polysaccharide is, you can click on the link, and the very first sentence of that article will tell you that "Polysaccharides are relatively complex carbohydrates." --Slashme 05:41, 17 October 2007 (UTC)
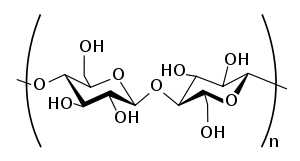
Serious Problem with Structure (possibly)
Please correct me if I'm wrong (I'm fairly new to sugars and polysaccharides and cyclic conformers bring me out in a nasty rash) but I'm fairly sure that the structure of cellulose is wrong - the structure shown isn't a polymer of Beta-D-glucose. The 3-hydroxyl should be equatorial and pointing above the plane (as currently oriented). Also the current structure that shows is not only a different polymer it's also conformationally impossible - if the 3-hydroxyl was pointing below the plane (as drawn) it would be axial not equatorial. Again, please forgive me if I'm wrong - I don't know if maybe the conformation is distorted by the hydrogen bonding(?) and that's what's been drawn.
I've just been back on the page and it looks like the 3D structure that's also shown and the diagram showing the hydrogen bonding between chains are also both wrong. I suspect the space filling model is also wrong but I can't get my head around the image to tell. Sorry to just put a post on here and not change it myself - I don't have access to a decent chem drawing program and I wouldn't want to change it without someone else confirming it's wrong. User:130.88.136.167
- This user may have a point, current image suggests a number three axial hydroxyl group where it should equatorial
alternative (also from commons):
The space filling model appears to show O-O bonds V8rik (talk) 20:32, 3 December 2008 (UTC)
I have exchanged the structure on the page with  which appears correct. We need to check the space filling and hydrogen bond pictures as well. AxelBoldt (talk) 19:11, 14 February 2009 (UTC)
which appears correct. We need to check the space filling and hydrogen bond pictures as well. AxelBoldt (talk) 19:11, 14 February 2009 (UTC)
I've found a few papers on the crystal structure(s) of cellulose:
Biomacromolecules, 2008, 9 (11), pp 3133–3140
Macromolecules, 2006, 39 (18), pp 6125–6132
Carbohydrate Research (2005) 340, 827-833
in Japanese, 2003, inaccessible to me
Russ. Chem. Rev. (1987) 56, 764
Journal of Applied Polymer Science (1974) 18 3373-3377
I've had a quick look at them and will try to make a model soon. There appear to be several crystalline modifications of cellulose - which one should we picture?
Ben (talk) 13:35, 15 February 2009 (UTC)
Maybe the Iβ conformation would be best as it is the predominant form in higher plants. Here's a good page about cellulose structure: [1]. AxelBoldt (talk) 18:25, 15 February 2009 (UTC)
- Ball-and-stick model of the crystal structure of cellulose Iβ added.
"Typically" polymers ?
I was wondering why the phrase
Plastics are typically polymers of high molecular mass, and may contain ...
contains the word typically, as if there are plastics that are not polymers. I am not aware of any plastic that is not also a polymer. Comments ? Stefano Borini (talk) 13:06, 29 July 2010 (UTC)