Galileo thermometer: Difference between revisions
Mr.Kennedy1 (talk | contribs) m Reverted edits by 24.106.188.82 (talk) to last revision by ClueBot (HG) |
m →Typical design: using math tags |
||
| Line 7: | Line 7: | ||
The temperature is typically read from an engraved metal disc on each bulb. Usually a gap separates the top bulbs from the bottom bulbs and then the temperature would be between the tag readings on either side of the gap. If a bulb is free-floating in the gap, then its tag reading would be closest to the ambient temperature. |
The temperature is typically read from an engraved metal disc on each bulb. Usually a gap separates the top bulbs from the bottom bulbs and then the temperature would be between the tag readings on either side of the gap. If a bulb is free-floating in the gap, then its tag reading would be closest to the ambient temperature. |
||
To achieve satisfactory accuracy, the weights are required to be manufactured to a tolerance of less than 1 |
To achieve satisfactory accuracy, the weights are required to be manufactured to a tolerance of less than <math>1\over 1000</math> of a [[gram]] (1 milligram).<ref>[http://www.thermometershop.co.uk/more_about____.htm#how How to read a Galilean Thermometer]</ref><ref>[http://hewgill.com/galilean-thermometer/ Galilean Thermometer]</ref><ref>[http://www.howstuffworks.com/question663.htm Howstuffworks]</ref> |
||
== Theory of operation == |
== Theory of operation == |
||
Revision as of 14:12, 6 September 2010

A Galileo thermometer (or Galilean thermometer), named after Italian physicist Galileo Galilei, is a thermometer made of a sealed glass cylinder containing a clear liquid and a series of objects whose densities are such that they rise or fall as the temperature changes.
Typical design
Suspended in the liquid are a number of weights. Commonly those weights are themselves attached to sealed glass bulbs containing colored liquid for an attractive effect. As the liquid in the cylinder changes temperature its density changes, and the bulbs are free to move, rising or falling to reach a position where their density is either equal to that of the surrounding liquid or where they are brought to a halt by other bulbs. If the bulbs differ in density by a very small amount and are ordered such that the least dense is at the top and most dense at the bottom, they can form a temperature scale..
The temperature is typically read from an engraved metal disc on each bulb. Usually a gap separates the top bulbs from the bottom bulbs and then the temperature would be between the tag readings on either side of the gap. If a bulb is free-floating in the gap, then its tag reading would be closest to the ambient temperature.
To achieve satisfactory accuracy, the weights are required to be manufactured to a tolerance of less than of a gram (1 milligram).[1][2][3]
Theory of operation

The Galilean thermometer works due to the principle of buoyancy. Buoyancy determines whether objects float or sink in a liquid, and is responsible for the fact that even boats made of steel can float (of course, a solid bar of steel by itself will sink). The only factor that determines whether a large object will rise or fall in a particular liquid relates the object's density to the density of the liquid in which it is placed. If the object's mass is greater than the mass of liquid displaced, the object will sink. If the object's mass is less than the mass of liquid displaced, the object will float.

Suppose there are two objects, each a cube 10 cm by 10 cm by 10 cm (i.e., 1 litre). The mass of water displaced by an object of this size is 1 kg. The brown object on the left is floating because the mass of water it would displace if completely submerged (1 kg) is greater than the mass of the object. It floats half submerged because that is the point where the mass of the water displaced (0.5 kg) is equal to the mass of the object. The green object on the right has sunk because the mass of water it is displacing (1 kg) is less than the object's mass (2 kg).

Not all objects made of the green material above will sink. In Figure 2, the interior of the green object has been hollowed out. The total mass of the object is now 0.5 kg, yet its volume remains the same, so it floats half way out of the water like the brown object in Figure 1.
In the examples above, the liquid in which the objects have been floating is assumed to be water. Water has a density of 1 kg/L, which means that the mass of water displaced by any of the above objects when fully submerged, is 1 kg.
Galileo discovered that the density of a liquid is a function of its temperature [1]. This is the key to how the Galileo thermometer works; as the temperature of water increases or decreases from 4oC, its density decreases. [2]
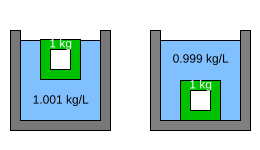
Figure 3 shows a 1 kg hollow object made of the green material. In the left hand container, the density of the liquid is 1.001 kg/L. Since the object weighs less than the mass of water it displaces, it floats. In the right hand container, the density of the liquid is 0.999 kg/L. Since the object weighs more than the mass of the water it displaces, it sinks. This shows that very small changes in the density of the liquid can easily cause an almost-floating object to sink.
In the Galileo thermometer, the small glass bulbs are partly filled with a different (coloured) liquid. Once the handblown bulbs have been sealed, their effective densities are adjusted by means of the metal tags hanging from beneath them. The expansion due to the temperature change of the coloured liquid and air gap (called ullage) inside the bulbs would affect their density were it not for the almost fixed volume of the glass bulb. The clear liquid in which the bulbs are submerged is not water, but some inert hydrocarbon (probably chosen because its density varies with temperature more than water does). This change of density of the clear liquid, with temperature change, causes the bulbs to rise or sink.

Figure 4 shows a schematic representation of a Galileo thermometer at two different temperatures (the temperature markings on this example are in Fahrenheit).
If there are some bulbs at the top (Figure 4, left) and some at the bottom, but one floating in the gap, then the one floating in the gap (green 76o) tells the temperature. If there is no bulb in the gap (Figure 4, right) then the average of the values of the bulb above and below the gap gives the approximate temperature. The bulbs and weights should be sized so as not to jam with each other, either by being at least half the size of the tube diameter to retain their stacking order or, as an alternative, much less than the tube diameter to freely pass each other in the tubes.

