Talk:Fuel cell: Difference between revisions
→MCFC Proposal: ce |
|||
| Line 344: | Line 344: | ||
:Looks good. I made some minor changes to simplify and to coordinate the section with the SOFC section (you don't need to repeat links that are given in the prev. section). Please check what I did and see if you have any questions. -- [[User:Ssilvers|Ssilvers]] ([[User talk:Ssilvers|talk]]) 03:36, 10 August 2011 (UTC) |
:Looks good. I made some minor changes to simplify and to coordinate the section with the SOFC section (you don't need to repeat links that are given in the prev. section). Please check what I did and see if you have any questions. -- [[User:Ssilvers|Ssilvers]] ([[User talk:Ssilvers|talk]]) 03:36, 10 August 2011 (UTC) |
||
::I appreciate your feedback. I looked at the edits you made, and noticed a few grammatical errors so I fixed them. Also, in the sentence starting "The main disadvantage of MCFC technology..." I changed the word disadvantage back to challenge. I think the "disadvantage" this sentence is referring to is really more of a challenge, since it is just a matter of finding the best materials to use to make the cell more durable. It is not a permanent disadvantage of the systems. The word challenge indicates that this is an area where researchers are actively working to overcome this challenge. -- [[User:LhamillFC|LhamillFC]] ([[User talk:LhamillFC|talk]]) 16:04, 11 August 2011 (UTC) |
|||
Revision as of 16:04, 11 August 2011
| This is the talk page for discussing improvements to the Fuel cell article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: Index, 1, 2, 3, 4Auto-archiving period: 90 days |
| This article is of interest to the following WikiProjects: | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
Index
|
||||
|
This page has archives. Sections older than 90 days may be automatically archived by Lowercase sigmabot III when more than 5 sections are present. |
| This is the talk page for discussing improvements to the Fuel cell article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google (books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: Index, 1, 2, 3, 4Auto-archiving period: 90 days |
SOFC contradiction
when talking about solid oxide fuel cells the example used with methanol is actually proton exchange membrane fuel cell. I have come to this conclusion because the description suggests that after methanol is catalytically broken up the H+ produced transfers to react with oxygen to create water. Solid oxide fuel cells do not do this. They transfer O2- ions from the oxygen side to react with methanol, as it says both in this article and the main SOFC article: http://en.wikipedia.org/wiki/Solid_oxide_fuel_cell
I would correct myself, but I don't know an appropriate example to replace this glaring error with and I don't want to cause any similar errors.
Efficiency Section
The Efficiency section is a pure copy paste from another source and may be a product of original research, suggest complete rewrite to conform to wikipedia standards. — Preceding unsigned comment added by Daniellis89 (talk • contribs) 22:11, 25 January 2011 (UTC)
- 180.149.16.211 (talk) 11:53, 21 May 2011 (UTC)what is the maximum efficiency of a fuel cell??
I would like to insert the following chart from the Department of Energy's, Energy Efficiency and Renewable Energy Fuel Cell Technology Program as I found that it offered a clear explanation of the different efficiencies of fuel cells, and may help clarify some of the other text in this section I found difficult to get though. Any advice on how best to size it?
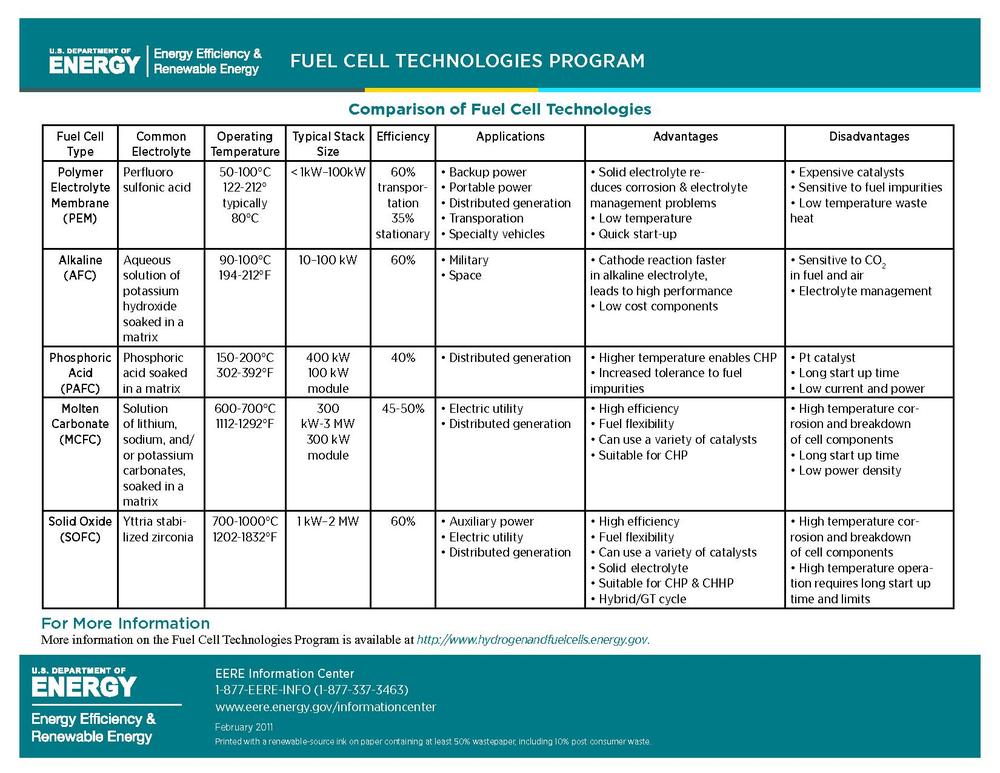
BBfchea 16:51, 1 August 2011 (UTC) — Preceding unsigned comment added by Briannabesch (talk • contribs)
You need a key defining all the terms used in the chart. You can find many of them here: http://www1.eere.energy.gov/hydrogenandfuelcells/glossary.html -- Ssilvers (talk) 23:19, 1 August 2011 (UTC)
- What terms exactly are you thinking of? I'd be happy to do this, but it feels redundant to re-define the types of fuel cell because I found them pretty well defined in the "types of fuel cell" section, and the rest seems pretty self explanatory. I was thinking people would know the term "electrolyte" and "Aqueous", and most of electrolytes are elements and therefore can't really be "defined". Let me know what you found unclear and I would be happy to define it. BBfchea (talk) 21:14, 2 August 2011 (UTC)
- Hi, Briana. Yes, you should include a glossary under the chart showing all the terms used in the chart so that readers do not have to look back and forth to other sections. Most encyclopedia readers have no background at all about fuel cells, and so they need to be able to easily understand the terms used in the chart; they may not realize that these terms are discussed in other sections and not know where to look. Our readers certainly do not know what "electrolyte" and "Aqueous" mean. They are just people who are curious about what a fuel cell is. As I said, you should write as if you are explaining this to a high school science class on the first day of school. -- Ssilvers (talk) 02:57, 3 August 2011 (UTC)
- Alright, I think I got everything that could potentially be confusing in the glossary now- let me know if you see terms I missed. I just considered most of these terms general chemistry, or in some cases every day life, terms so didn't really see the need to define them here, but hopefully the glossary I created will be helpful to those who have absolutely no background in science. I used Merriam-Webster online dictionary definitions for ones not found in the DOE EERE Fuel Cell Technologies program glossary. — Preceding unsigned comment added by Briannabesch (talk • contribs) 13:43, 3 August 2011 (UTC)
Applications Section
I found a lot of out-dated information in this section that I am going to update.
Power Section- The fuel cell market has expanded a lot in the last few years, and I am updated and expanding the information in this section to reflect these developments. Stationary fuel cells have also been used by many different companies in addition to Stuart Island, so I will add in more examples.
Combined Heat and Power- Information is out of date and there are few citations (many are broken or now re-directed), I am updating information and including more recent sources.
Hydrogen transportation and refueling- This title isn't very clear, I will change to Fuel Cell Transportation Vehicles and Hydrogen Refiling,
Land Vehicles- As there are many different types of fuel cell land vehicles I will change title to Fuel Cell Electric Vehicles (FCEVs) as this is the name the industry generally refers to when talking about cars. Most of the information in this section is not relevant to FCEVs and is out of date. I will take out old information and update the section with recent numbers from the Department of Energy. (DOE) There have also been a lot more FCEVs developed in the last few years, I will add more recent developments from major auto manufacturers.
Aircraft, Boats and Submarines- I will add to these section to include more recent developments
Fuel Cell Forklifts- One of the major Fuel Cell applications is Forklifts for the material handling industry, I will create a section that talks about forklifts.
Fueling Stations- A lot of this information is out of date with broken links, I will update and include more up-to-date sources.
Market Structure- I will take this section out of applications and combine it with Fuel Cell Economics as that seems to make more seance.
Briannabesch (talk) 19:31, 22 July 2011 (UTC)
Proton Exchange Membrane Fuel Cells
There are several pieces of information in this section of the Fuel Cell Wikipedia page that I found to be either out of date, not cited, or wrong. I would like to change these facts on the page.
Currently on Wikipedia: "In 2002, typical fuel cell systems cost US$1000 per kilowatt of electric power output." Correct statement: "In 2002 projected improvements in performance and operation on hydrogen led to an estimate of approximately $100/kW for the system cost" Source: http://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/tiax_cost_analysis_pres.pdf
Currently on Wikipedia: "In 2008 UTC Power has 400 kW stationary fuel cells for $1,000,000 per 400 kW installed costs" Correction: UTC does not list their prices, and there are many different payment options, tax incentives, etc. so I don't think we should include a price on the Wikipedia page. It is misleading.
Currently on Wikipedia: "The production costs of the PEM (proton exchange membrane). The Nafion membrane currently costs $566/m²" Correction: In 2005, NREL studies showed that with the average cost of Nafion at $80/lb, a Nafion membrane would cost about $23/m^2. The high end of membrane cost is about $27/m^2. Source: http://www.nrel.gov/hydrogen/pdfs/39104.pdf
LhamillFC (talk) 19:41, 22 July 2011 (UTC)
SOFC
This is a great section, but there are certain areas that need more explanation.
Currently on Wikipedia: "A major disadvantage of the SOFC, as a result of the high heat, is that it 'places considerable constraints on the materials which can be used for interconnections'." The source for this fact is out of date... There is much research being conducted on SOFCs that are dealing with these problems, so a source from 2010 (11 years ago) does not seem like it should be included.
Currently on Wikipedia: "Another disadvantage of running the cell at such a high temperature is that other unwanted reactions may occur inside the fuel cell. It is common for carbon dust (graphite) to build up on the anode, preventing the fuel from reaching the catalyst." Again... there is much research being done on SOFCs including research that addresses this problem. I think that this information: "The anode or fuel side electrode typically is composed of a cermet-containing YSZ and Ni metal. The Ni metal acts as a catalyst for the oxidation of the fuel. Among the problems faced with a Ni cermet anode is Ni metal coarsening (sintering) during use, as well as the buildup of carbon deposits on the surface (coking) during internal reforming of the fuel. Researchers at the University of Pennsylvania have shown that the use of copper (Cu)-based cermets dramatically reduces coking and loss of performance during service due to coking." should be included Source: http://www.ceramicindustry.com/Articles/Feature_Article/10637442bbac7010VgnVCM100000f932a8c0____
Currently on Wikipedia: "Much research is currently being done to find alternatives to YSZ that will carry ions at a lower temperature." There is also research being done to reduce the temperature while still using YSZ. Source: http://www.sciencedirect.com/science/article/pii/S0378775308002243
LhamillFC (talk) 20:21, 22 July 2011 (UTC)
Not only is there research being done to find alternatives to YSZ, but there are also alternatives currently being used in fuel cell systems being sold today. Ceres Power's SOFC that they sell uses CGO (cerium gadolinium oxide) as the electrolyte instead of YSZ, allowing operating temperature to drop to 500-600 degrees C. I would like to add this into the section. LhamillFC (talk) 13:00, 3 August 2011 (UTC)
- I agree that you should update the research in this section, as long as you provide a balanced discussion, noting where there is any disagreement in the major sources. Old statements that are no longer true can be replaced by current information, unless they provide some useful historical information. The fact that a reference is old does not *necessarily* mean that it is useless. -- Ssilvers (talk) 13:22, 3 August 2011 (UTC)
MCFC
This section explains a fundamental aspect of how MCFCs work. I think it makes it seem like a problem that needs to be sorted out "somehow". The DOE has a good explanation of this, and I think it should replace what is currently on Wikipedia.
"Because the electrolyte loses carbonate in the oxidation reaction, the carbonate must be replenished through some means. This is often performed by recirculating the carbon dioxide from the oxidation products into the cathode where it reacts with the incoming air and reforms carbonate."
DOE explanation: "At the anode, hydrogen reacts with the carbonate ions to produce water, carbon dioxide, and electrons. The electrons travel through an external circuit creating electricity and return to the cathode. There, oxygen from the air and carbon dioxide recycled from the anode react with the electrons to form carbonate ions that replenish the electrolyte and provide ionic conduction through the electrolyte, completing the circuit."
Source: http://www.fossil.energy.gov/programs/powersystems/fuelcells/fuelcells_moltencarb.html LhamillFC (talk) 21:00, 22 July 2011 (UTC)
- Can you translate any of this high temperature section into English so that a non-technical person can at least understand the nature of what is being discussed? Otherwise, 99% of readers will simply skip the section. -- Ssilvers (talk) 13:30, 3 August 2011 (UTC)
- Are you talking about the DOE definition I included at the beginning of the section?LhamillFC (talk) 15:04, 3 August 2011 (UTC)
- Yes, because this is something that you added. But in general, if you can translate any of the technical stuff to plainer English, it would be a major improvement to the article. For guidance, see #7 and #8 here. -- Ssilvers (talk) 15:35, 3 August 2011 (UTC)
Socking in this discussion
I have blocked LhamillFC (talk · contribs), Briannabesch (talk · contribs), and Pfchea (talk · contribs) as confirmed socks of Connordfc (talk · contribs). Keegan (talk) 07:43, 28 July 2011 (UTC)
- After discussion by email I have unblocked the accounts. They are individuals working from the same place. They are conditionally unblocked that they follow WP:COI and refrain from discussing changes to fuel cell related articles with each other and use the talk page process to work with other editors. WP:AGF. Keegan (talk) 21:09, 29 July 2011 (UTC)
Market Structure and Economics
Hi all, I wanted to to combine the "Market Structure" section under "Fuel Cell Applications" with the Economics section as that seemed to fit better under economics then applications, and then would change the "economics" title to "Fuel Cell Markets and Economics". The first sentence under the current "Market Structure" section is not cited, and the second has a broken link, I would like to take this sentence out unless someone ells knows where this information can be found and cited (let me know and I'll put it in!). I felt the second sentence of the "Economics" section, "A fuel cell and electric motor combination is not directly limited by the Carnot efficiency of an internal combustion engine." should be taken out as this point is addressed in the "efficiency" section of the page. I also wanted to add in more infomration about the current fuel cell market around the world, so the first paragraph would then read:
"In 2010, fuel cell industry revenues exceeded a $750 million market value[1] and 0.14 million unit shipments, with a average annual growth rate of 115%. [2] Aproximately 50% of fuel cell shipments in 2010 were stationary fuel cells, up from about a third in 2009.[3] The "Big Four" players in the Fuel Cell Industry remain the United States, Germany, Japan and South Korea.[4] Current stationary fuel cells can generate power at approximately $724 to $775 per kilowatt installed.[5] Translated to a consumer, this means stationary fuel cells can generate power at 9-11 cents per kilowatt-hour, including the price of fuel, maintenance, and hardware.[6] A typical stationary fuel cell will meet its return on investment in 3-5 years.[7] Hydrogen is a candidate as a storage mechanism and can help ease the integration of renewable energy generation into our existing grid. We can produce hydrogen though distributed electrolysis generation wherever and whenever excess electricity is produced. This hydrogen can then be distributed to where it is needed, to be turned back into electricity to meet peak demand or even power FCEVs. In this way hydrogen becomes a keystone in the creation of an alternative energy future and a hydrogen economy.
I'll leave this up here for a day before changing- let me know what you think!BBfchea 16:21, 1 August 2011 (UTC) — Preceding unsigned comment added by Briannabesch (talk • contribs)
- I changed the heading for you and deleted the old "markets" section, as you suggested. Headings do not need to repeat the name of the article (Fuel Cell). Also, headings should start with an initial capital letter, but the other words in the heading should not be capitalized, unless it is a proper name. In your refs, you are still missing a lot of necessary bibliographical information. You need to give the author's name (where available), and always give the title of the article (put the url before the title in brackets like this:[url "Title"]) and the name of the publisher (and a page number if from a book or long article). Then give the date published (if available) and the accessdate. The <ref> tag should follow the punctuation with no space. In general, we should write as if we are explaining our subject to an intelligent person with no background in chemistry, engineering or investing, who does not know what a fuel cell is. Another idea is to pretend you are a high school science teacher or economics teacher, as you write, explaining this technology on the first day of school.
- Some comments on your suggested paragraph above: "...2010 revenues exceeding $750 million and 0.14 million unit shipments, with a average annual growth rate of 115%". This sentence is not meaningful. Does it mean for mobile applications, stationary applications, or both? Worldwide? What is a "unit shipment"? You need to give context, since we are writing for a general readership encyclopedia. Also, Pike Research is a marketing firm. So, its bias is to try to raise money for whatever technology it is writing about. So this is not a "neutral" source, and we need to introduce their conclusions with something like, "According to Pike Research, a market research and consulting firm engaged by the fuel cell industry to conduct a market study...." You wrote above: "...stationary fuel cells can generate..." Don't tell us what fuel cells "can do", tell us what they actually do in the field, and back up your assertion with a citation to an independent WP:Reliable source that states clearly the fact and its context, such as how they tested that info. Bloom Energy is not a WP:Reliable source, since it is a commercial supplier advertising its product. We should not refer to company websites for assertions about the performance of those companys' products. You need to use independent (preferably peer reviewed) studies. If we must refer to a company's website for anything, we have to say that "according to Bloom Energy, which sells stationary fuel cells", or something like that. Look at this sentence: "We can produce hydrogen though distributed electrolysis generation wherever and whenever excess electricity is produced." Who is "we"? This begs several questions: What is the cost of "distributed electrolysis generation" compared with hydrogen reforming? How much CO2 does each produce directly and indirectly? What does "distributed" mean in that phrase? "Wherever and whenever" is what we call a WP:PEACOCK phrase. What you mean is "where". But where is there "excess electricity"? This is very markety, rather than encyclopedic writing. Moreover, what do these last five unreferenced sentence have to do with markets and economics? They belong elsewhere, in an explanation of what a stationary fuel cell is, and what its purpose is.
- We must explain to our readers what things are and how they work. For an example of a good marketing explanation, see this. More generally, for some examples of good articles about technologies, see, for example: Atomic line filter and Shale oil extraction. Good luck! -- Ssilvers (talk) 18:34, 1 August 2011 (UTC)
Thanks for your comments, two heads are always better than one. Below I have posted the new first paragraph, but I first wanted to address a couple of things SSilvers had said.
-I have now formatted sources to be more consistent with the articles previous sources. Several of the sources do not have authors, but I included as much information as I could find.
-About Pike Research, they are a market research firm, not a marketing firm- their mission statement is
- “Pike Research is an independent market research firm whose goal is to present an objective, unbiased view of market opportunities within its coverage areas. The firm is not beholden to any special interests and is thus able to offer clear, actionable advice to help clients succeed in the industry, unfettered by technology hype, political agendas, or emotional factors that are inherent in cleantech markets.”
I actually found the numbers and facts I cite from Pike Research ($750 million market value, 50% stationary fuel cell shipments, Germany, Japan, USA and South Korea as the countries with the largest market shares) in the paragraph on a number of news sources first, and though it was most responsible to cite the original published study. As such I consider it a WP:Reliable Source.
-I’m sorry about the confusing wording with “can”, I have changed it to reflect that fuel cells do generate power at $724-$775 per kW installed and 9-11 cents per kW consumer price. I also put in that Bloom Energy stationary fuel cells achieve a 3-5 year payback.
-Hydrogen is also being looked at as a storage mechanism, I significantly revised this section to take into account your comments. I can continue to look to update the sources as well. I would also be ok moving this last section to the "application section" if people feel that that would work better.
This is the revised paragraph I would like to put in.
In 2010, fuel cell industry revenues exceeded a $750 million market value worldwide[8]. There were 0.14 million fuel cell stacks shipped globally in 2010, up from 11 thousand shipments in 2007; in 2010 worldwide fuel cell shipments had an annual growth rate of 115%. [9] Approximately 50% of fuel cell shipments in 2010 were stationary fuel cells, up from about a third in 2009.[10] The "Big Four" players in the Fuel Cell Industry remain the United States, Germany, Japan and South Korea.[11] The Department of Energy Solid State Energy Conversion Alliance found that, as of January 2011, stationary fuel cells generated power at approximately $724 to $775 per kilowatt installed.[12] Bloom Energy, a major fuel cell supplier, says its fuel cells will meet a return on investment in 3-5 years, translated to a consumer, this means stationary fuel cells generate power at 9-11 cents per kilowatt-hour, including the price of fuel, maintenance, and hardware.[13] [14]
Another emerging market for hydrogen is a mechanism to store energy, particularly excess energy from intermittent renewable energy sources like solar and wind farms. [15] As most renewable energy sources are intermittent, storage mechanisms must be devised to provide reliable power (see Grid energy storage). The National Renewable Energy Laboratory is conducting the wind-to-hydrogen project: this project is testing ways to use the electricity produced by wind mills or solar panels when electricity demand is low to electrolyze water and form hydrogen. [16] That hydrogen can then be stored, and turned back into electricity via a fuel cell when electricity demand is high.[17] This application is particularly important for places hoping to rely on intermittent renewable power.
BBfchea 21:25, 1 August 2011 (UTC) — Preceding unsigned comment added by Briannabesch (talk • contribs)
As people haven't commented on this in the last 36 hours I'm going to go ahead and add this section in now — Preceding unsigned comment added by Briannabesch (talk • contribs) 13:52, 3 August 2011 (UTC)
- Good work. I cleaned up your references. Remember that the author name (last, first) goes at the beginning. I deleted the paragraph on hydrogen storage, as that did not seem to be about fuel cells. Perhaps it would be useful in some other article. All the best! -- Ssilvers (talk) 14:35, 3 August 2011 (UTC)
Incomplete references
User:Pfchea, User:Connordfc, User:LhamillFC, and User:Briannabesch, thank you for the additions that you made to this entry last week. Please fill out the incomplete references that you have added to this entry with author names, article titles, publisher names, publication dates and, where available, page numbers. I have left notes on some of your talk pages about how to do this, and the relevant guideline is WP:CITE. Please let me know if you need more assistance. Thanks! -- Ssilvers (talk) 15:27, 2 August 2011 (UTC)
- Thanks for notice user:Ssilvers. We are actively working to properly cite any sources we have linked on this page. After reviewing the References section, I found that most of the citations on the page are simply links, not just those included by my colleagues. In addition, there is not much conformity between the References, it seems pretty haphazard for the most part, and some of them are actually dead links. As an additional project, we will work to edit all of the citations on this page so that they are consistent, and active. Thanks for bringing this matter to our attention. Connordfc (talk) 18:03, 2 August 2011 (UTC)
- Looks like you have made good progress. I will check later and clean up any remaining formatting issues. I note that the author name should always go first, with the author's last name. Here is the basic format: Twain, Mark. [url A Connecticut Yankee in King Arthur's Court]. Publisher name, date, page number, ISBN#, access date. Best regards, -- Ssilvers (talk) 19:35, 2 August 2011 (UTC)
- Thanks, I'v almost the rest of the page with all the info I could find. Ssilvers If you wouldn't mind clarifying your comments about defining terms in the chart I proposed inserting under the efficiency section I would appreciate it. BBfchea (talk) 21:19, 2 August 2011 (UTC)
- You guys have done excellent work cleaning up this aricle. May I recomend using
{{cite book}}and{{cite web}}for formatting references. It guarrentees consistency. WP:wikicite makes life easier for you too. Cheers. Stepho talk 23:01, 2 August 2011 (UTC)
- You guys have done excellent work cleaning up this aricle. May I recomend using
- Wow, Brianna. Good work. See above re the chart. BTW, I moved the bus and forklift discussions a bit higher, right under cars. As for ref style, I like book template, but personally, I think the cite web template just makes the refs harder to work with. -- Ssilvers (talk) 03:16, 3 August 2011 (UTC)
Introduction to the article
I found that the introduction to the article was rather confusing and technical. I wrote an alternative that I tried to make more straight foreword and accessible to the common reader. Thoughts?
A fuel cell is a device that produces electricity through an electrochemical process. It converts the chemical energy stored in a fuel into electricity through a chemical reaction with an oxidizing agent, usually oxygen. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are also used. In addition to direct current electricity fuel cells produce water, heat and, depending on the fuel source, sometimes carbon dioxide. Fuel Cells are different from batteries in that they require a constant source of fuel and oxidizing agent to run, but can produce electricity continually for as long as these inputs are supplied.
Welsh PhysicistWilliam Grove developed the first crude fuel cell, based off a concept developed by his fellow scientist and friend Christian Friedrich Schonbein in 1939. The first commercial use of fuel cells was in NASA space programs to generate power for probes, satellites and shuttles. [18] Since then fuel cells have been used in many other applications. Fuel cells are used to power buildings, many different types vehicles, and to charge smaller electronic devices like laptops and smartphones.
There are many types of fuel cells, and they are each classified by the fuel cell’s electrolyte, the substance that allows charges to move within the fuel cell. Fuel cells come in a variety of sizes. Individual fuel cells only produce very small amounts of electricity, so cells are ‘stacked’, or placed in series, to increases the voltage output to meet application’s power generation requirements. BBfchea (talk) 17:18, 3 August 2011 (UTC)
- Good idea. Here is the guideline on introduction sections: WP:LEAD. Basically, the lead should give an overview of the whole article, touching on the most important points that will be discussed below. With a long article like this, it should be three or four paragraphs long. You don't need to repeat refs in the lead, as long as it is just summarizing things that are referenced below. BTW, in Wikipedia, we always use " and ' rather than curly quotes and apostropies. See WP:MOS You made a very good start, and I would simplify it even further, like this:
A fuel cell is a device that converts the chemical energy stored in a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used. Fuel Cells are different from batteries in that they require a constant source of fuel and oxygen to run, but they can produce electricity continually for as long as these inputs are supplied. Welsh Physicist William Grove developed the first crude fuel cells in 1839. The first commercial use of fuel cells was in NASA space programs to generate power for probes, satellites and space capsules. Since then, fuel cells have been used in many other applications. Fuel cells are used to power buildings, many different types of vehicles, and to charge smaller electronic devices like laptops and smartphones.
There are many types of fuel cells, which are classified by the fuel cell's electrolyte, the substance that allows charges to move within the fuel cell. Fuel cells come in a variety of sizes. Individual fuel cells only produce very small amounts of electricity, so cells are "stacked", or placed in series, to increases the voltage output to meet application’s power generation requirements. In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, sometimes carbon dioxide, nitrogen dioxide and very small amounts of other emissions.
- As I said, a very good start, and I think you need to add to this a bit more "overview" information summarizing a few more of the high points in the article below. -- Ssilvers (talk) 20:58, 3 August 2011 (UTC)
- Thanks! I went through the fuel cell article and tried to add in some more points to your suggestions. If this seems alright to everyone I would like to go ahead and add it soon. How does this sound?
A fuel cell is a device that converts the chemical energy stored in a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used. Fuel Cells are different from batteries in that they require a constant source of fuel and oxygen to run, but they can produce electricity continually for as long as these inputs are supplied.
Welsh Physicist William Grove developed the first crude fuel cells in 1839. The first commercial use of fuel cells was in NASA space programs to generate power for probes, satellites and space capsules. Since then, fuel cells have been used in many other applications. Fuel cells are used to generate primary and backup power for commercial, industrial and residential applications as well as for remote and or inaccessible areas. They are used to power fuel cell vehicles, including automobiles, busses, forklifts, airplanes, boats, motorcycles and submarines.
There are many types of fuel cells, but they all consist of an anode (negative side), cathode (positive side) and electrolyte that allows charges to move between the two sides. Charges are pushed from the anode to the cathode, though an external circuit which creates usable direct current electricity. As the main difference between fuel cells types is the electrolyte, fuel cells are classified by the type of electrolyte they use. Fuel cells come in a variety of sizes. Individual fuel cells only produce very small amounts of electricity, about 0.7V, so cells are "stacked", or placed in series or parallel circuits, to increase the voltage and current output to meet an application’s power generation requirements.[19] In addition to electricity, fuel cells produce water, heat and, depending on the fuel source, sometimes carbon dioxide, and very small amounts of nitrogen dioxide and other emissions. The energy efficiency of a fuel cell is generally between 40-60%, 85% effcient if waste heat is captured for use, [20] which is significantly greater than a typical combustion power generation process. [21] [22]
- Thanks. This is a good step forward. I made some minor changes and put it into the Lead section. I removed the last sentence, because it would be better not to get into the comparisons of other technologies until we get into the body of the article where there is room for a fuller explanation. -- Ssilvers (talk) 06:05, 5 August 2011 (UTC)
Efficiency: theory section - Proposed Overhaul
So I am trying to tackle the information that is written in the efficiency section. The current sections of "Theory" and "In Practice" I found difficult to understand, out of date, not well referenced and occasionally factually wrong. I attempted to re-write the Theory section, keeping in as best I could the information that was cited (and the links weren't dead) and making it sound more accessible. This is what I came up with- let me know what you think!
- There are many different ways to measure efficiencies. Any time you convert energy from one form to another you lose some of the energy. An energy efficiency value represents the ratio between useful output energy and total input energy or what percentage of the total energy put in the system that is retained though the energy conversion. In the case of fuel cells, useful output energy is measured in electrical energy produced by the system. Input energy refers to the total energy stored in the fuel. Fuel cells are generally between 40-60% energy efficient. [23] This is significantly higher than some other forms of energy generation. For example, the typical internal combustion engine of a car is only about 25% energy efficient. [24] In combined heat and power (CHP) systems, the heat produced by the fuel cell is captured and put to use, increasing the efficiency of the system to 85-90%. [20]
- As there will be losses when converting one form of energy into another, it is possible to calculate the theoretical maximum efficiency of any type of power generation. While these values are rarely reached in practice, this can be a useful exercise to compare different types of power generation. Under typical operating conditions the maximum theoretical amount of energy efficiency of a fuel cell is 83% (not including any gains from captured waste heat, as is done in heat and power co-generation). [25] This is significantly more than a combustion engine, which has a maximum theoretical efficiency of 58%.[26] While these efficiencies are not usually approached in real life, but high temperature fuel cells (solid oxide fuel cells or molten carbonate fuel cells) can be combined with gas turbines to approach this theoretical limit. The gas turbine captures heat from the fuel cell and turns it into mechanical energy that increases the fuel cell’s operational efficiency. This solution increases total efficiency to an "ultrahigh" level of 70%.[27]
I'll be working on the "in practice" section next BBfchea (talk) 21:09, 3 August 2011 (UTC)
Also: Under the "In practice" section it was posted that "the overall efficiency (electricity to hydrogen and back to electricity) of such plants (known as round-trip efficiency) is between 30 and 50%, depending on conditions." However the source cited- a NASA Study entitled Round Trip Energy Efficiency of NASA Glenn Regenerative Fuel Cell System says "NASA Glenn Research Center (GRC) has recently demonstrated a Polymer Electrolyte Membrane (PEM) based hydrogen/oxygen regenerative fuel cell system (RFCS) that operated for a charge/discharge cycle with round trip efficiency (RTE) greater than 50 percent." There is no mention of 30% anywhere I found- I would propose taking this out unless the 30% can be shown to be taken from elsewhere BBfchea (talk) 21:23, 3 August 2011 (UTC)
- (To clarify I mean just the 30% number, not the whole quote)BBfchea (talk) 12:50, 4 August 2011 (UTC)
Since it has been about 24 hours I'm going to go ahead and post this- let me know if you have any questions BBfchea (talk) 21:11, 4 August 2011 (UTC)
- Good. I made some minor edits and filled in some refs more. The NASA article concludes on p. 5, 35% to 50%. Also, the study was done with pure oxygen rather than air. I have clarified. -- Ssilvers (talk) 07:20, 5 August 2011 (UTC)
- I see, I was searching for 30% in the article which is why I didn't look at the 35%. After further looking though I found that the article also says "The average cell voltage of the fuel cell throughout the discharge cycle was 0.80 V which, compared to the 1.48 V thermo neutral point, leads to an operating efficiency of 54 percent." If its alright I would like to change the 30-50% to 35-55%. I know its a small thing, but since we have the correct info it seems like it should be changed. -- BBfchea (talk) 19:10, 5 August 2011 (UTC)
- I don't think we should make too much of this study, because they used pure oxygen instead of air, and this section is supposed to be about practical results. This was not the "conclusion", so the reporter must have thought that the 54% reading was anomalous. I think it would be cherry-picking, since it's not mentioned in the conclusion, so I'd suggest that we not change it and stick with what they reported in the conclusion. -- Ssilvers (talk) 19:27, 5 August 2011 (UTC)
SOFC proposal
I agree that the high temperature section is not organized very well, too technical and sometimes even false (see "SOFC contradiction" above). I have rewritten the SOFC section and will post it here for comments.
- Solid oxide fuel cells use a solid material, most commonly a ceramic material called yttria-stabilized zirconia (YSZ), as the electrolyte. Because SOFCs are made entirely of solid materials, they are not limited to the flat plane configuration of other types of fuel cells and are often designed as rolled tubes. They require high operating temperatures (800-1000 degrees Celsius), and can be run on a variety of fuels including natural gas.[28]
- SOFCs are unique in that negatively charged oxygen ions travel from the cathode (negative side of the fuel cell) to the anode (positive side of the fuel cell) as opposed to positively charged hydrogen ions travelling from the anode to the cathode, as is the case in all other types of fuel cells. Oxygen gas (O2) is fed through the cathode where the molecule reacts with electrons to create oxygen ions (O-2). The oxygen ions then travel through the electrolyte to react with hydrogen gas (H2) at the anode. The reaction at the anode produces electricity and water as by-products. Carbon dioxide (CO2) may also be a by-product depending on the fuel, but the carbon emissions from an SOFC system are much less than those from a fossil fuel combustion plant[29]. The chemical reactions for the SOFC system are found below[30]:
- Anode Reaction: 2H2 + 2O–2 => 2H2O + 4e–
- Cathode Reaction: O2 + 4 e– => 2O–2
- Overall Cell Reaction: 2H2 + O2 => 2H2O
- SOFC systems are fuel-flexible, meaning they can run on fuels other than pure hydrogen gas. However, since hydrogen gas is necessary for the reactions listed above, the fuel of choice must contain hydrogen atoms. In order for the fuel cell to operate, the fuel must be reformed to produce pure hydrogen gas. SOFCs are capable of internally reforming light hydrocarbons such as methane (natural gas), propane, and butane. Heavier hydrocarbons including gasoline, diesel, jet fuel and biofuels can serve as fuels in a SOFC, but an external reformer is required.
- Certain challenges exist in SOFC systems due to their high operating temperatures. One such challenge is the potential for carbon dust to build up on the anode, which slows down the internal reforming process. Research addressing this “carbon coking” issue at the University of Pennsylvania has shown that the use of copper-based cermet (heat-resistant materials made of ceramic and metal) dramatically reduces coking and loss of performance during service due to coking.[31]
- Another challenge posed by the high operating temperature is slow start up time for SOFC systems, making SOFCs not ideal for mobile applications. The high operating temperature is largely due to the physical properties of the YSZ electrolyte. As temperature decreases, so does the ionic conductivity of YSZ. Therefore, to obtain optimum performance of the fuel cell, a high operating temperature is required.
- Ceres Power, UK SOFC fuel cell manufacturer, discovered a way to reduce the operating temperature of their SOFC system to 500-600 degrees Celsius. They replaced the commonly used YSZ electrolyte with a CGO (cerium gadolinium oxide) electrolyte. The lower operating temperature allows them to use stainless steel instead of ceramic as the cell substrate, which reduces cost and start up time of the system.[32]
- Although there is the disadvantage of slow start up time, a high operating temperature does provide advantages as well. The high temperature removes the need for a precious metal catalyst like platinum, thereby reducing cost. Additionally, waste heat from SOFC systems may be captured and reused, increasing the overall efficiency to 80%-85%.[33]
LhamillFC (talk) 14:05, 4 August 2011 (UTC)
This looks good. I made some minor copy edits to the section. Nicely done! -- Ssilvers (talk) 17:58, 5 August 2011 (UTC)
Fuel Cell Vehicle
A couple of edits for the fuel cell vehicles section. I would like to propose the removal of the last paragraph of the Fuel Cell Vehicle section, below, as it does not relate to fuel cell vehicles. This paragraph would maybe fit under an article on the history of hydrogen, or on the full fuel cell vehicle page under history, but to me doesn't seem to fit in the "applications" section of the Fuel Cell page.
- In 2003 US President George Bush proposed the Hydrogen Fuel Initiative (HFI), which was later implemented by legislation through the 2005 Energy Policy Act and the 2006 Advanced Energy Initiative. These aimed at further developing hydrogen fuel cells and infrastructure technologies with the goal of producing commercial fuel cell vehicles. By 2008, the U.S. had contributed 1 billion dollars to this project.[68] In May 2009, however, the Obama Administration announced plans to "cut off funds" for the development of fuel cell vehicles, concluding that other vehicle technologies will lead to quicker reduction in emissions in a shorter time. Steven Chu, the US Secretary of Energy, asserted that hydrogen vehicles "will not be practical over the next 10 to 20 years".[69] He told MIT's Technology Review that he is skeptical about hydrogen's use in transportation because of four problems: "the way we get hydrogen primarily is from reforming [natural] gas. ... You're giving away some of the energy content of natural gas. ... [For] transportation, we don't have a good storage mechanism yet. ... The fuel cells aren't there yet, and the distribution infrastructure isn't there yet. ... In order to get significant deployment, you need four significant technological breakthroughs.[70] The National Hydrogen Association and the U.S. Fuel Cell Council criticized this decision.[71] Congress reversed the funding cuts in its appropriations bill for 2010,[72] but the Department of Energy plans to decrease funding for Fuel Cell Vehicle development by 41% in its 2012 budget.[73]
I would also like to change the third paragraph.
- -I think it would be appropriate to move the first sentence about well-to-wheel analysis up to the second paragraph as it contains as it is not talking about challenges like the rest of the paragraph. Changing the second sentence to "some analysis" instead of "other analysis, however, found.." follows from this change.
- -I would like to propose an update to the quote currently from the article. The current quotation is from a news article about a study done by Ulf Bossel, however out of the context of the study the quote previously put in could be misread. The study done was only on completely renewable production of hydrogen, as opposed to the 95% of hydrogen that is currently reformed from natural gas. I would like to replace the quote from the news article with one from the actual study itself, which I believe better represents the research that was done. I could not figure out the second reference for the intial quote, as that was a completely separate study that did not look at same type of energy loss or quote anything like 25% energy loss for hydrogen- I would propose removing this citation as it does not seem to apply to anything in the previous paragraph, of the edits I am proposing.
- -I added in some more information to the last paragraph
- Fuel cell electric vehicles have been produced with "a driving range of more than 400 km (250 mi) between refueling".[34] They can be refueled in less than 5 minutes.[35] EERE’s Fuel Cell Technology Program claims that, as of 2011, fuel cells achieved a 42 to 53% fuel cell electric vehicle efficiency at full power,[36] and a durability of over 120,000 km (75,000 mi) with less than 10% degradation, double that achieved in 2006.[34]. In a Well-to-Wheels analysis, the U.S. Department of Energy estimated that fuel cell electric vehicles using hydrogen produced from natural gas would result in emissions of approximately 55% of the CO2 per mile of internal combustion engine vehicles and have approximately 25% less emissions than hybrid vehicles.[37]
- Still, challenges remain before fuel cell cars can become economically competitive with other technologies. Analysis cite the lack of an extensive hydrogen infrastructure in the U.S. as an ongoing challenge to Fuel Cell Electric Vehicle commercialization. In 2006, a study for the IEEE showed that in hydrogen produced via electrolysis of water using only renewable means: "Only about 25% of the power generated from wind, water, or sun is converted to practical use." [38] Some experts believe that it would take at least 20 years for manufacturers to achieve profitable production.[39] Still, Pike Research predicted that there will be over one million hydrogen vehicles and 5,200 hydrogen refueling stations globally by 2020. [40][41]
74.82.102.4 (talk) 18:45, 8 August 2011 (UTC) BBfchea (talk) 21:24, 8 August 2011 (UTC)
The George Bush paragraph is an essential part of the history. Please do not delete it, although it could be streamlined. Also, I think the Ulf Bossel material is correct in context. The point being made has nothing to do with the method of production. You must clearly state all assumptions made, when you discuss technology development and studies. Punctuation always must go before the ref tags, not afterwards. Other changes will be reviewed and revised when they are added to the article. Make sure that the changes do not try to whitewash the criticism of this technology. Are you one of the editors from the Fuel Cell and Hydrogen Energy Association? You forgot to log in. All the best, -- Ssilvers (talk) 19:05, 8 August 2011 (UTC)
- Sorry, I had signed the post but didn't realized I wasn't logged in at the time, sorry about that. I added my signature above, but the time stamp will be slightly off. For the paragraph I proposed removing my thinking was that it is, as you said, history, and as such I don't think it fits quite right in the "applications" section of the page. As the fuel cell vehicle page has expanded I was thinking it might be better suited under there- if you disagree I understand that, and will attempt to stream line but would also appreciate some other opinions. Also, although the administration said it would cut off fuel cell funding they haven't done that, fuel cell budgets have been cut but so has the entire Department of Energy's budget. A 2012 budget hasn't yet been passed in the Senate, but the House passed a budget that cut fuel cell programs from $100,000 to ~$91,000, a much smaller cut that was made to many other programs, including Solar and Wind research, so saying that the DOE proposed cuts of 41% cuts is not telling the whole story.
- About the quote- the way that the hydrogen is obtained has a lot to do with the well-to-wheels energy- it is much more inefficient to make hydrogen though electrolysis of water then it is to reform it from natural gas. That is why solid oxide fuel cell systems can reach 60% inefficiencies, starting from Natural Gas Fuel. The quote is correct in saying that in 2006 you only recovered about 25% of the energy you started with when you make hydrogen from electrolysis and transport it etc. But this is not the case when you make hydrogen from natural gas, or on site, and this efficiency may have improved when you take into account some of the work NREL has done on electrolysis of hydrogen (which I haven't had a chance to look up yet). I am not trying to whitewash criticism- its true, fuel cell cars aren't currently ready for mass production, we don't have the infrastructure in place, but that does not mean that they don't have an important role to play in an alternative transportation portfolio, and to only talk about past criticism and not mention recent developments is also not neutral. That's also why I try and post everything in discussion first- to allow others to express their views or disagree and make sure my comments fairly represent what is going on. I'll leave this in discussion until tomorrow, in case any one ells would like to comment, and then post it with your edits in mind. BBfchea (talk) 21:24, 8 August 2011 (UTC)
I've streamlined the paragraph. The reason I put it with the Automobile section is that it is about cars. It doesn't affect the other parts of the article. But it is, I think, essential here. -- Ssilvers (talk) 22:41, 8 August 2011 (UTC)
- I'm repeating myself here, but I disagree with you about Bossel. The quote used in the article here takes into account natural gas reforming. Moreover, it is totally misleading to talk about production on site: We do NOT have hydrogen filling stations that make hydrogen on site - you have to compress it and truck it in expensively. Also, making hydrogen from natural gas creates emissions. So, the Bossel quote is absolutely necessary here to balance our very aggressive use of the well-to-wheels analysis, which doesn't even mention the emissions created, and which is merely an estimate, rather than a real-world study. You are correct that we must mention the most recent and important developments; but we must clearly describe their context and show the assumptions made. -- Ssilvers (talk) 23:10, 8 August 2011 (UTC)
- We must be careful not to give readers the impression that these technologies are more mature than they are – developing manufacturing technologies have always been prone to setbacks and often slow to become commercially viable. In an encyclopedic article we should be cautious in asserting that a new technology is likely to have a significant commercial effect, because we don't yet know how likely it is. Tim riley (talk) 06:43, 9 August 2011 (UTC)
- this is BBfchea (talk) 16:38, 9 August 2011 (UTC)I don't know why I can't sign at the bottom
- To Tim riley- thanks for weighing in- I have tried to keep my revisions to studies done about fuel cell vehicles by reputable sources, and hadn’t though I had talk to much about their future commercial impact, but if you have any specific sugestions let me know.
- To SSilvers: Thanks for streamlining the paragraph on the funding of FCEVs, its more readable now, and if you feel it’s important to keep in here I guess that’s fine. Could we integrate this paragraph to the one on the fuel cell vehicle page as well? I though this one contains more information than the one currently there. I also wanted to replace the statement that the National Hydrogen Association and Fuel Cell Council disagreed with secretary Chu's statements, as these two organizations merged to form the Fuel Cell and Hydrogen Energy Association early this year (see www.fchea.org) and the citation is a broken link. I would like to replaced it with a quote about the Secretary's onions from the Mary Nichols, Chairwomen of California's Air Resources Board:However, others disagree with Secretay Chu's opinions- Mary Nichols, Chairwomen of California's Air Resources Board stated "Secretary Chu has firmly set his mind against hydrogen as a passenger-car fuel...Frankly, his explanations don’t make sense to me. They are not based on the facts as we know them.” [42]
- I would like to point out that I did mention CO2 emission in the automobile section, specifically that they emit: “55% of the CO2per mile of internal combustion engine vehicles and have approximately 25% less emissions than hybrid vehicles”. I am happy to put the number of grams/mile the cars emit if that’s helpful (260 for FCEVs, 340 for Hybrids, 470 for combustion vehicles). I actually have found a demonstration study done by Argon National Lab, GM and Air Improvement Resource, Inc that showed FCEVs running on hydrogen produced from natural gas emit 250 g/mile of GHGs well-to-wheel (FCEV emit not emissions locally) while a displacement on demand conventional spark ignition vehicle produces 550 g/mile of GHGs well-to-wheel which are pretty in line with the numbers predicted by the DOD. I will replace the numbers from the DOD Study with these (although it is from 2005 I couldn’t find anything more recent that weren’t projections). I can also add in that the study found FCEVs running on gracious natural gas use ~4,000 BTU/mile (well-to-wheel) while conventional Displacement on demand conventional vehicles use ~6,500 BTU/mile (well-to-wheel).
- There are actually a significant number of hydrogen stations that produce hydrogen on site in the U.S. Fuel Cells 2000 keeps a list of hydrogen filling stations in the U.S. and abroad (http://www.fuelcells.org/info/charts/h2fuelingstations.pdf). Some of the stations that produce hydrogen on site include stations in Port Hueneme, CA, Rosemead, CA, Sacramento, CA, Sata Monica, CA, Torrance, CA, Detroit, Mi, Selfridge, Mi, Two in Las Vegas, NV, White Plains, NY, Columbus, OH, Topton PA, University Park, PA, Austin, TX and others, both domestically and internationally. Some of these produce hydrogen directly via electrolysis on site, others are hooked into the natural gas pipeline and reform it onsite. Although it is limited, hydrogen pipelines do exists, in May 2011 Toyota announced the opening of the first fueling station to get hydrogen directly by pipeline (http://pressroom.toyota.com/releases/toyota+hydrogen+fueling+station+may+2011.htm)- so while some hydrogen is delivered by truck a significant amount is not, and for future fueling stations developing getting hydrogen on site is certainly an option. However, I didn’t mention any of that in the paragraphs I’m proposing potion on the page, I was just trying to convey to you that you can’t say getting hydrogen from any source, though any transportation mechanism, to run though any fuel cells, will have the same efficiency- efficiencies very widely depending on what path you take, which is why WTW analysis are so tricky.
- We continue to disagree about the quote “"the large amount of energy required to isolate hydrogen from natural compounds (water, natural gas, biomass), package the light gas by compression or liquefaction, transfer the energy carrier to the user, plus the energy lost when it is converted to useful electricity with fuel cells, leaves around 25% for practical use." I believe that the news article is not correctly quoting Bossel’s research. For starters, nowhere ells except that tile sentence does article does it mention making hydrogen natural gas. The diagram showed is specifically talking about the production of hydrogen via electrolysis from water.
- My though is that Bossel’s original study would be more reliable then the news article. The study can be found here, http://www.efcf.com/reports/E21.pdf. It is entitled “Does a Hydrogen Economy Make Sense” was published in October 2006 for the Institute of Electrical & Electronics Engineers, Inc. The study is on “sustainable” hydrogen production, which excludes natural gas “In this paper, fossil and nuclear energy are defined as unsustainable because the resources are finite and the waste cannot be absorbed by nature.” (pg 1827). The study looks at “ Production of Hydrogen by Electrolysis” and “Hydrogen From Biomass” (pg 1828) but does not look at natural gas reforming. The study states concludes “Only about 25% of the power generated from wind, water, or sun is converted to practical use.” He also says “Industrial natural gas reformers generate hydrogen with energetic HHV efficiencies of 90%. Today, this is the most economical method to obtain hydrogen. As stated earlier, hydrogen production from fossil hydrocarbons is not here considered sustainable.”
- I concluded that as the original study only talks about renewable sources of hydrogen generation, and that should be stated in the main Wikipedia article- I am fine quoting Bossel to say that when you generate hydrogen from renewable resources, either through a process of water electrolysis or obtaining it from biomass, only 25% of the energy you start with ends up as electricity you can use practically, I don't know if this has changed in the last 6 years, but if I come across more information from reputable sources will suggest and update.
- After all of this discussion, I would like to propose inserting the following in place of the - hopefully we have cleared up any disagreement.
- Fuel cell electric vehicles have been produced with "a driving range of more than 400 km (250 mi) between refueling".[34] They can be refueled in less than 5 minutes.[43] EERE’s Fuel Cell Technology Program claims that, as of 2011, fuel cells achieved 53-59% efficiency at ¼ power and 42-53% vehicle efficiency at full power,[36] and a durability of over 120,000 km (75,000 mi) with less than 10% degradation, double that achieved in 2006.[34] In a Well-to-Wheels analysis, it was found that a 2005 FCEV running on compressed gracious hydrogen produced from natural gas used ~4,000 BTUs/mile and emitted ~250 grams/mile of Green House Gasses. This is about 40% less energy and 45% less emissions then an internal combustion vehicle which was found to use ~6,500 BTU/mile and emitted ~550 g/mile of green house gasses well-to-wheel. [44]
- Still, challenges remain before fuel cell cars can become economically competitive with other technologies. Analysis cite the lack of an extensive hydrogen infrastructure in the U.S. as an ongoing challenge to Fuel Cell Electric Vehicle commercialization. In 2006, a study for the IEEE showed that renewable hydrogen produced via electrolysis of water using renewable electricity or reformed from biomass found: "Only about 25% of the power generated from wind, water, or sun is converted to practical use." [45] Fuel cell vehicles running on renewable created hydrogen produce almost no CO2 or greenhouse gas emissions.Some experts believe that it would take at least 20 years for manufacturers to achieve profitable production.[39] Still, Pike Research predicted that there will be over one million hydrogen vehicles and 5,200 hydrogen refueling stations globally by 2020. [46] [47]
More on Fuel Cell Vehicle
Hi, BB. I think this is worse than what is there now. This now adds in even more numerical stuff, with mathematical symbols, that is irrelevant to readers of a general encyclopedia. It also confuses the issues between cars and other vehicles. The way that the Pike research estimates are used is highly misleading. See what Tim riley said above and read WP:CRYSTAL - predictions are not very useful. In 2003, President Bush and GM said that there would be hundreds of thousands of FC cars on the road by 2010. Now, GM's CEO says that he thinks there will not be any commercial production until "2020 plus". Plus, Pike talks about things other than cars. My advice is that if you want to replace information, try to do it in plain English that readers with no science or math background can understand, and try to stick to what is actually known from real-world testing, not just predictions about what might happen if and when there is volume production of hydrogen cars. Very importantly: make sure that you note what is for cars and what is for something else. See the Meyers article that is cited for a good explanation. All the best. -- Ssilvers (talk) 16:59, 9 August 2011 (UTC)
- Hi again. So I'm assuming your ok with all of the other points I addressed earlier in that post (the CO2, Fueling Stations that produce hydrogen on site and the quote from Mary Nichols and the Bossel study). After looking up the WP:CRYSTAL I had decided that since Pike was a reputable source that talked about the "arguments about the prospects for success of future proposals and projects or whether some development will occur," it would be ok to put in, especially to go alongside "Experts believe that it would take at least 20 years for manufacturers to achieve profitable production" to make sure both viewpoints of fast and slow development were represented- but took it out given the issues you raised. I also worked to streamline the section with all of these edits.
- I don't think that mentioning how much energy the car uses per mile or how much green house gas it emits is to technical- if people are confused they can clink to the Wikipedia link to BTU they will see it is an energy unit, not a 'mathematical symbol'. These numbers add weight to some of the things that fuel cell vehicles do and are therefore quite important to the page. I have tried to clarify this sentence to make it more understandable, but many Wikipedia articles state some basic math, numbers and science terms (e.g. the featured articles on Acetic acid, the Big Bang and Cell Nucleus) These terms shouldn't be excessive but I believe a middle schooler could undrestand "emitted 250 grams per mile of greenhouse gasses"
- No, I do not agree that a significant number of fueling stations are actually producing H2 on site. Some of them say that they will do so in the future, but when? One or two actually say that they already do so, but they only produce enough H2 to fuel a handful of cars per day. I suggest that you do not delete referenced information from this section, unless you set forth here the exact language that you want to delete so we can discuss it. As I said, whatever you add, please try to phrase it in plain English. Do not assume that I agree with anything you have said, unless I say so explicitly, because you are writing too much to respond to. If you feel that an addition should be made, make it, and then other editors will respond to it. All the best, -- Ssilvers (talk) 19:22, 9 August 2011 (UTC)
- Just trying to be thorough, and comply with WP:Conflict of Interest guidelines. I don't believe I'm deleting anything I haven't thoroughly discussed here, although a few things are re-organized for clarity. Thanks for clarifying my proposal- I want to replace the current Automobile section with the one below- if there are not other edits in the next 24 hours I'll post this :)
- BBfchea (talk) 20:09, 9 August 2011 (UTC)
BB proposal: Although there are currently no Fuel cell vehicles available for commercial sale, over 20 FCEVs prototypes and demonstration cars have been released since 2009.[48] As of June 2011 demonstration FCEVs had driven more than 4,800,000 km (3,000,000 mi), with more than 27,000 refuelings.[49] Automobiles such as the Honda FCX Clarity, Toyota FCHV-adv, and Mercedes-Benz F-Cell are all pre-commercial examples of fuel cell electric vehicles. Still, some experts believe that it would take at least 20 years for manufacturers to achieve profitable production.[39] In July 2011, the Chairman and CEO of General Motors, Daniel Akerson, stated that while the cost of hydrogen fuel cell cars is decreasing: "The car is still too expensive and probably won't be practical until the 2020-plus period, I don't know."[50] Additionally, many major car manufacturers have announced plans to introduce a production model of a fuel cell car in 2014-2015. Toyota has stated that it plans to introduce such a vehicle at a price of around US$50,000.[51] In June 2011, Mercedes-Benz announced that they would move the scheduled production date of their fuel cell car from 2015 up to 2014:[52] Dr. Dieter Zetsche, chairman of the board of management of Daimler and head of Mercedes-Benz Cars stated "The product is ready for the market technically," but noted that the remaining issue is infrastructure, which partners needed to take the lead on. [53]
Fuel cell electric vehicles have been produced with "a driving range of more than 400 km (250 mi) between refueling".[34] They can be refueled in less than 5 minutes.[54] EERE’s Fuel Cell Technology Program claims that, as of 2011, fuel cells achieved 53-59% efficiency at ¼ power and 42-53% vehicle efficiency at full power,[36] and a durability of over 120,000 km (75,000 mi) with less than 10% degradation, double that achieved in 2006.[34] In a Well-to-Wheels analysis, it was found that, per mile traveled, a fuel cell electric vehicle running on compressed gaseous hydrogen produced from natural gas used about 40% less energy and emit 45% less green house gasses then an internal combustion vehicle. Well-to-Wheel the Fuel Cell Vehicle on average used approximately 4,000 British Thermal Units (BTUs) of energy per mile it drove, and emitted approximately 250 grams/mile of green house gas emissions, while a conventional car used approximately 6,500 BTU/mile and emitted approximately 550 grams/mile of green house emissions when taking all the energy needed to make the car run, well-to-wheels. [55]
Still, challenges remain before fuel cell cars can become economically competitive with other technologies. Analysis cite the lack of an extensive hydrogen infrastructure in the U.S. as an ongoing challenge to Fuel Cell Electric Vehicle commercialization. In 2006, a study for the IEEE showed that renewable hydrogen produced via electrolysis of water using renewable electricity or reformed from biomass found: "Only about 25% of the power generated from wind, water, or sun is converted to practical use." [56] Fuel cell vehicles running on renewable created hydrogen produce almost no CO2 or greenhouse gas emissions.
In 2003 US President George Bush proposed the Hydrogen Fuel Initiative (HFI). This aimed at further developing hydrogen fuel cells and infrastructure technologies with the goal of producing commercial fuel cell vehicles. By 2008, the U.S. had contributed 1 billion dollars to this project.[57] The Obama Administration has sought to reduce funding for the development of fuel cell vehicles, concluding that other vehicle technologies will lead to quicker reduction in emissions in a shorter time.[58] Steven Chu, the US Secretary of Energy, asserted that hydrogen vehicles "will not be practical over the next 10 to 20 years".[59] He told MIT's Technology Review that he is skeptical about hydrogen's use in transportation because of four problems: "the way we get hydrogen primarily is from reforming [natural] gas. ... You're giving away some of the energy content of natural gas. ... [For] transportation, we don't have a good storage mechanism yet. ... The fuel cells aren't there yet, and the distribution infrastructure isn't there yet. ... In order to get significant deployment, you need four significant technological breakthroughs.[60] However, others disagree with Secretay Chu's opinions- Mary Nichols, Chairwomen of California's Air Resources Board stated "Secretary Chu has firmly set his mind against hydrogen as a passenger-car fuel...Frankly, his explanations don’t make sense to me. They are not based on the facts as we know them.” [61] BBfchea (talk) 18:54, 9 August 2011 (UTC)
MCFC Proposal
As I did with the SOFC section, I have rewritten the MCFC section to make it more comprehensive. I plan to write a section on each type of fuel cell using the same structure as I did with the SOFC and MCFC section. My hope is to create uniformity and make it easier for people to pick out specific information about fuel cells and compare information among the different types of fuel cells. Below is my MCFC draft - edits are welcome before I post it on the page.
- Molten carbonate fuel cells (MCFCs) are similar to solid oxide fuel cells (SOFCs), in that they require a high operating temperature (650 degrees Celsius). The difference between the two types of fuel cell is found in the electrolyte. MCFCs use lithium potassium carbonate salt as an electrolyte, and at high temperatures, this salt melts into a molten state that allows for the movement of charge (in this case, negative carbonate ions) within the cell.[62]
- Like SOFCs, MCFCs are capable of internally reforming fuel in the anode, eliminating the need for an external reformer. MCFC-compatible fuels include natural gas, biogas and gas produced from coal. Once the fuel is reformed into a hydrogen-rich gas, hydrogen reacts with carbonate ions from the electrolyte to produce water, carbon dioxide and electrons. The electrons travel through an external circuit creating electricity and return to the cathode. There, oxygen from the air and carbon dioxide recycled from the anode react with the electrons to form carbonate ions that replenish the electrolyte, completing the circuit.[63] The chemical reactions for an MCFC system can be expressed as follows:[64]
- Anode Reaction: CO3-2 + H2 => H2O + CO2 + 2e-
- Cathode Reaction: CO2 + ½O2 + 2e- => CO3-2
- Overall Cell Reaction: H2 + ½O2 => H2O
- As with SOFCs, there is the issue of slow start up time that comes with high operating temperature. This makes MCFC systems not suitable for mobile applications, and this technology will most likely be used for stationary purposes.[citation needed]
- The main challenge of current MCFC technology is their short life span, which is a result of the high operating temperature and corrosive electrolyte. These factors accelerate the degradation of MFCF components, decreasing the durability and cell life. Researchers are addressing this problem by exploring corrosion-resistant materials for components as well as fuel cell designs that increase cell life without decreasing performance.[65]
- Although there is the issue of cell degradation, MCFCs hold several advantages over other fuel cell technologies. One advantage is their resistance to impurities. They are not prone to “carbon coking”, which refers to reduced performance through the build-up of carbon slowing down the internal reforming process. Because there is not the problem of carbon coking, carbon-rich fuels like gases made from coal are compatible with the system. The Department of Energy (DOE) claims that coal, itself, might even be a fuel option in the future, assuming the system can be made resistant to impurities such as sulfur and particulates that result from converting coal into hydrogen.[66]
- MCFCs are also much more efficient than other kinds of fuel cells. They can reach a fuel-to-electricity efficiency of 50%, considerably higher than the 37-42% efficiency of a phosphoric acid fuel cell plant. Efficiencies can be as high as 65% when the fuel cell is paired with a turbine and 85% when heat is captured and used in a Combined Heat and Power (CHP) system.[67]
- FuelCell Energy, a Connecticut-based fuel cell manufacturer, develops MCFC technology and sells their products on a global scale. Their MCFC products range from 300kW to 2.8MW systems that all achieve 47% electrical efficiency and utilize CHP technology to obtain higher overall efficiencies. One product, the DFC-ERG, is combined with a gas turbine and achieves an electrical efficiency of 65%.[68]
LhamillFC (talk) 17:51, 9 August 2011 (UTC)
Sounds good. Try to combine the short little paragraphs together (putting related ideas together in the same paragraph), so that the section is just a few longer paragraphs. That makes it read more smoothly and look nicer. -- Ssilvers (talk) 19:25, 9 August 2011 (UTC)
- Looks good. I made some minor changes to simplify and to coordinate the section with the SOFC section (you don't need to repeat links that are given in the prev. section). Please check what I did and see if you have any questions. -- Ssilvers (talk) 03:36, 10 August 2011 (UTC)
- I appreciate your feedback. I looked at the edits you made, and noticed a few grammatical errors so I fixed them. Also, in the sentence starting "The main disadvantage of MCFC technology..." I changed the word disadvantage back to challenge. I think the "disadvantage" this sentence is referring to is really more of a challenge, since it is just a matter of finding the best materials to use to make the cell more durable. It is not a permanent disadvantage of the systems. The word challenge indicates that this is an area where researchers are actively working to overcome this challenge. -- LhamillFC (talk) 16:04, 11 August 2011 (UTC)
- ^ Pike Research,Fuel Cell Industry is Poised for Major Change and Development in 2011, Published Feb 2nd 2011, accessed August 1 2011, http://www.pikeresearch.com/newsroom/fuel-cell-industry-is-poised-for-major-change-and-development-in-2011
- ^ Global Fuel Cell Market by Technology, Application, Component,Installation, Cost, Geography, Trends and Forecasts (2011 – 2016), May 2011,http://www.marketsandmarkets.com/Market-Reports/fuel-cell-market-348.html, accessed August 1 2011
- ^ Pike Reaserch, Fuel Cell Annual Report 2011, Published 2Q 2011, Karry-Ann Adamson, Ph.D and Clint Wheelock,http://www.pikeresearch.com/wordpress/wp-content/uploads/2011/05/FCAR-11-Executive-Summary.pdf, accessed August 1 2011
- ^ Pike Research, Fuel Cells Annual Report 2011, pg 3, Published Q2 2011,http://www.pikeresearch.com/wordpress/wp-content/uploads/2011/05/FCAR-11-Executive-Summary.pdf, Kerry-Ann Adamson, Ph.D. and Clint Wheelock, Accessed August 1st 2011
- ^ Solid State Energy Conversion Alliance, SECA Cost Reduction, Updated January 31st 2011, Accessed August 1 2011, http://www.fossil.energy.gov/programs/powersystems/fuelcells/fuelcells_seca.html
- ^ Bloom Energy Plays the Subsidy Game Like a Pro, by Eric Wesoff, Published April 13th 2011, Accessed August 1 2011, http://www.wired.com/epicenter/tag/bloom-energy/
- ^ Bloom Energy, Lower & Lock-In Energy Costs, http://bloomenergy.com/benefits/lower-fix-energy-costs/
- ^ http://www.pikeresearch.com/newsroom/fuel-cell-industry-is-poised-for-major-change-and-development-in-2011, Fuel Cell Industry is Poised for Major Change and Development in 2011, Pike Research, Published Feb 2nd 2011, accessed August 1 2011,
- ^ http://www.marketsandmarkets.com/Market-Reports/fuel-cell-market-348.html, Global Fuel Cell Market by Technology, Application, Component, Installation, Cost, Geography, Trends and Forecasts (2011 – 2016), May 2011, Markets and Markets, accessed August 1 2011
- ^ http://www.pikeresearch.com/wordpress/wp-content/uploads/2011/05/FCAR-11-Executive-Summary.pdf, Fuel Cell Annual Report 2011, Published 2Q 2011, Karry-Ann Adamson, Ph.D and Clint Wheelock, Pike Research, accessed August 1 2011
- ^ http://www.pikeresearch.com/wordpress/wp-content/uploads/2011/05/FCAR-11-Executive-Summary.pdf, Fuel Cells Annual Report 2011, pg 3, Kerry-Ann Adamson, Ph.D. and Clint Wheelock, Pike Research, Published Q2 2011, Accessed August 1st 2011
- ^ http://www.fossil.energy.gov/programs/powersystems/fuelcells/fuelcells_seca.html, Solid State Energy Conversion Alliance SECA Cost Reduction section, Updated January 31st 2011, Accessed August 1 2011,
- ^ http://bloomenergy.com/benefits/lower-fix-energy-costs/, Bloom Energy, Lower & Lock-In Energy Costs
- ^ http://www.wired.com/epicenter/tag/bloom-energy/, Bloom Energy Plays the Subsidy Game Like a Pro, Eric Wesoff, Published April 13th 2011, Accessed August 1 2011,
- ^ http://www.areva.com/EN/operations-408/hydrogen-and-fuel-cells.htm, Hydrogen: adapting to the demand for energy, AREVA
- ^ http://www.nrel.gov/hydrogen/proj_wind_hydrogen_video.html, Wind-to-Hydrogen Project Video, National Renewable Energy Labratory
- ^ http://www.nrel.gov/hydrogen/proj_wind_hydrogen.html, NREL, Wind-to-Hydrogen Project,7 July 2011, Accessed August 1 2011
- ^ Fitzgerald, Justin and O'Bryan, Nancy. "Fuel Cells: A Better Energy Source for Earth and Space". NASA. 2-11-2005
- ^ Nice, Karim and Strickland,Jonathan. "How Fuel Cells Work: Polymer Exchange Membrane Fuel Cells" How Stuff Works. Accessed August 4th 2011
- ^ a b Cite error: The named reference
www1.eere.energy.govwas invoked but never defined (see the help page). - ^ "Fuel Cell Efficiency". World Energy Council. 2011
- ^ "Energy Sources: Electric Power".U.S. Department of Energy. Accessed August 2nd 2011.
- ^ "Comparison of Fuel Cell Technologies". Department of Energy Energy Efficiency and Fuel Cell Technologies Program. February 2011.
- ^ "Fuel Economy: Where The Energy Goes". Department of Energy Energy Effciency and Renewable Energy www.fueleconomy.gov. August 03 2011. Accessed August 3nd 2011.
- ^ "Fuel Cell Efficiency". World Energy Council. 2011
- ^ "Fuel Cell Efficiency". World Energy Council. 2011
- ^ Milewski, J., Miller, A., Badyda, K. "The Control Strategy for High Temperature Fuel Cell Hybrid Systems". The Online Journal on Electronics and Electrical Engineering (OJEEE). Vol. (2)- No. (4). Pg331.
- ^ "Types of Fuel Cells". “Department of Energy EERE website, Retrieved on: 2011-08-04.
- ^ Stambouli, A. Boudghene. "Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy". “Renewable and Sustainable Energy Reviews, Vol. 6, Issue 5, Pages 433-455, October 2002.
- ^ >"Solid Oxide Fuel Cell (SOFC)". “FCTec website, Retrieved on: 2011-08-04.
- ^ Hill, Michael. "Ceramic Energy: Material Trends in SOFC Systems". “Ceramic Industry, September 1, 2005.
- ^ "The Ceres Cell". “Ceres Power website, Retrieved on: 2011-08-04.
- ^ "Types of Fuel Cells". “Department of Energy EERE website, Retrieved on: 2011-08-04.
- ^ a b c d e f "Accomplishments and Progress". Fuel Cell Technology Program, U.S. Dept. of Energy, June 24, 2011
- ^ Wipke, Keith, Sam Sprik, Jennifer Kurtz and Todd Ramsden. "National FCEV Learning Demonstration". National Renewable Energy Laboratory, April 2011, accessed August 2, 2011
- ^ a b c Garbak, John. "VIII.0 Technology Validation Sub-Program Overview". DOE Fuel Cell Technologies Program, FY 2010 Annual Progress Report, accessed August 2, 2011
- ^ "Distributed Hydrogen Production via Steam Methane Reforming". Well-to-Wheels Case Studies for Hydrogen Pathways, DOE Hydrogen Program, accessed August 2, 2011
- ^ Bossel, Ulf. "Does a Hydrogen Economy Make Sense? Proceedings of the IEEE Vol. 94, No. 10, October 2006.
- ^ a b c Cite error: The named reference
Meyers1was invoked but never defined (see the help page). - ^ "Fuel Cell Vehicle Sales to Cross the 1 Million Mark in 2020". Pike Research. April 19th 2011. Accessed August 8th 2011
- ^ [http://www.pikeresearch.com/newsroom/more-than-5200-hydrogen-fueling-stations-to-be-operational-by-2020 "More than 5,200 Hydrogen Fueling Stations to be Operational by 2020". Pike Research. July 19th 2011. Accessed August 8th 2011.
- ^ Ohnsman, Alan and Wingfield, Brian. "Obama Hydrogen Fuel Failure Conceded by Chu Paring Budget: Cars". Bloomberg. July 14, 2011. Accessed August 9 2011.
- ^ Wipke, Keith, Sam Sprik, Jennifer Kurtz and Todd Ramsden. "National FCEV Learning Demonstration". National Renewable Energy Laboratory, April 2011, accessed August 2, 2011
- ^ Brinkman, Norma, Wang, Michael, Weber, Trudy, Darlington, Thomas. "Well-To-Wheels Analysis of Advanced Fuel/Vehicle Systems- A North American Study of Energy Use, Greenhouse Gas Emissions, and Criteria Pollutant Emissions. General Motors Corporation, Argonne National Laboratory and Air Improvement Resource, Inc.. May 2005. Accessed August 9th 2011
- ^ Bossel, Ulf. "Does a Hydrogen Economy Make Sense? Proceedings of the IEEE Vol. 94, No. 10, October 2006.
- ^ "Fuel Cell Vehicle Sales to Cross the 1 Million Mark in 2020". Pike Research. April 19th 2011. Accessed August 8th 2011
- ^ [http://www.pikeresearch.com/newsroom/more-than-5200-hydrogen-fueling-stations-to-be-operational-by-2020 "More than 5,200 Hydrogen Fueling Stations to be Operational by 2020". Pike Research. July 19th 2011. Accessed August 8th 2011.
- ^ "Hydrogen and Fuel Cell Vehicles Worldwide". TÜV SÜD Industrie Service GmbH, accessed on August 2, 2011
- ^ Wipke, Keith, Sam Sprik, Jennifer Kurtz and Todd Ramsden. "Controlled Hydrogen Fleet and Infrastructure Demonstration and Validation Project". National Renewable Energy Laboratory, September 11, 2009, accessed on August 2, 2011
- ^ Shepardson, David. "GM CEO: Fuel cell vehicles not yet practical". The Detroit News, July 30, 2011
- ^ Squatriglia, Chuck. "Hydrogen Is Down, But Not Out". Wired.com, Autopia, March 17, 2011, accessed on August 2, 2011
- ^ Lienert, Anita. "Mercedes-Benz Fuel-Cell Car Ready for Market in 2014". Edmunds Inside Line, June 21, 2011
- ^ Lienert, Anita ."Mercedes-Benz Fuel-Cell Car Ready for Market in 2014" Edmunds Inside Line. June 21 2011. Accessed August 9 2011
- ^ Wipke, Keith, Sam Sprik, Jennifer Kurtz and Todd Ramsden. "National FCEV Learning Demonstration". National Renewable Energy Laboratory, April 2011, accessed August 2, 2011
- ^ Brinkman, Norma, Wang, Michael, Weber, Trudy, Darlington, Thomas. "Well-To-Wheels Analysis of Advanced Fuel/Vehicle Systems- A North American Study of Energy Use, Greenhouse Gas Emissions, and Criteria Pollutant Emissions. General Motors Corporation, Argonne National Laboratory and Air Improvement Resource, Inc.. May 2005. Accessed August 9th 2011
- ^ Bossel, Ulf. "Does a Hydrogen Economy Make Sense? Proceedings of the IEEE Vol. 94, No. 10, October 2006.
- ^ Nice, Karim, and Jonathan Strickland. "How Fuel Cells Work". How Stuff Works, accessed August 3, 2011
- ^ Chu, Steven. "Winning the Future with a Responsible Budget". U.S. Dept. of Energy, February 11, 2011
- ^ Matthew L. Wald (2009-05-07), U.S. Drops Research Into Fuel Cells for Cars, New York Times, retrieved 2009-05-09
- ^ Bullis, Kevin. "Q & A: Steven Chu", Technology Review, May 14, 2009
- ^ Ohnsman, Alan and Wingfield, Brian. "Obama Hydrogen Fuel Failure Conceded by Chu Paring Budget: Cars". Bloomberg. July 14, 2011. Accessed August 9 2011.
- ^ “Molten Carbonate Fuel Cell Technology”. “U.S. Department of Energy”, Retrieved on 2011-08-09.
- ^ “Molten Carbonate Fuel Cell Technology”. “U.S. Department of Energy”, Retrieved on 2011-08-09.
- ^ “Molten Carbonate Fuel Cells (MCFC)”. “FCTec”, Retrieved on 2011-08-09.
- ^ “Types of Fuel Cells”. “U.S. Department of Energy Office of Energy Efficiency and Renewable Energy”, Retrieved on 2011-08-09.
- ^ “Types of Fuel Cells”. “U.S. Department of Energy Office of Energy Efficiency and Renewable Energy”, Retrieved on 2011-08-09.
- ^ “Molten Carbonate Fuel Cell Technology”. “U.S. Department of Energy”, Retrieved on 2011-08-09.
- ^ “Products”. “FuelCell Energy”, Retrieved on 2011-08-09.


