Aortic aneurysm: Difference between revisions
reworded complicated sentences; reorganized paragraphs to be in logical order |
→Pathophysiology: Made statements more accurate |
||
| Line 57: | Line 57: | ||
==Pathophysiology== |
==Pathophysiology== |
||
[[File:6.5cmAAAwith3cmlumen.png|thumb|A 6.5cm AAA with a 3cm lumen]] |
[[File:6.5cmAAAwith3cmlumen.png|thumb|A 6.5cm AAA with a 3cm lumen]] |
||
An aortic aneurysm can occur as a result of trauma, infection, or, most commonly, from an intrinsic abnormality in the [[elastin]] and [[collagen]] components of the aortic wall. |
|||
==Prevention== |
==Prevention== |
||
Revision as of 16:46, 26 August 2013
| Aortic aneurysm | |
|---|---|
| Specialty | Vascular surgery |
An aortic aneurysm is a general term for an enlargement (dilation) of the aorta to greater than 1.5 times normal size.[1] While the cause of an aneurysm may be multifactorial, the end result is an underlying weakness in the wall of the aorta at that location. The aneurysm may occasionally cause pain, which is a sign of impending rupture. When rupture occurs, massive internal hemorrhage results, and, unless treated immediately, shock and death can occur within minutes to hours.
Classification
Aortic aneurysms are classified by their location on the aorta.
- An aortic root aneurysm, or aneurysm of the sinus of Valsalva.
- Thoracic aortic aneurysms are found within the chest; these are further classified as ascending, aortic arch, or descending aneurysms.
- Abdominal aortic aneurysms, the most common form of aortic aneurysm, are found on the abdominal aorta. Thoracoabdominal aortic aneurysms involve both the thoracic and abdominal aorta. There are other classifications that might help treatment.
Signs and symptoms
Most intact aortic aneurysms do not produce symptoms. As they enlarge, symptoms such as abdominal pain and back pain may develop. Compression of nerve roots may cause leg pain or numbness. Untreated, aneurysms tend to become progressively larger, although the rate of enlargement is unpredictable for any individual. Rarely, clotted blood which lines most aortic aneurysms can break off and result in an embolus.
Aneurysms can be found on physical examination. Medical imaging is necessary to confirm the diagnosis and to determine the anatomic extent of the aneurysm. In patients presenting with aneurysm of the arch of the aorta, a common sign is a hoarse voice from stretching of the left recurrent laryngeal nerve, a branch of the vagus nerve that winds around the aortic arch to supply the muscles of the larynx.
Abdominal aortic aneurysm
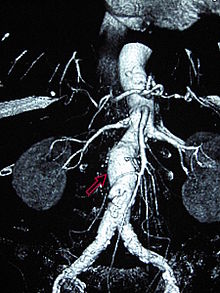
Abdominal aortic aneurysms (AAAs) are more common than their thoracic counterpart. One reason for this is that elastin, the principal load-bearing protein present in the wall of the aorta, is reduced in the abdominal aorta as compared to the thoracic aorta (nearer the heart). Another is that the abdominal aorta does not possess vasa vasorum. Most AAA are true aneurysms that involve all three layers (tunica intima, tunica media and tunica adventitia). The prevalence of AAAs increases with age, with an average age of 65–70 at the time of diagnosis. AAAs have been attributed to atherosclerosis, though other factors are involved in their formation.
The risk of rupture of an AAA is related to its diameter; once the aneurysm reaches about 5 cm, the yearly risk of rupture may exceed the risks of surgical repair for an average-risk patient. Rupture risk is also related to shape; so-called "fusiform" (long) aneurysms are considered less rupture prone than "saccular" (shorter, bulbous) aneurysms, the latter having more wall tension in a particular location in the aneurysm wall.
Before rupture, an AAA may present as a large, pulsatile mass above the umbilicus. A bruit may be heard from the turbulent flow in the aneurysm. Unfortunately, however, rupture may be the first hint of AAA. Once an aneurysm has ruptured, it presents with a classic pain-hypotension-mass triad. The pain is classically reported in the abdomen, back or flank. It is usually acute, severe and constant, and may radiate through the abdomen to the back.
The diagnosis of an abdominal aortic aneurysm can be confirmed at the bedside by the use of ultrasound. Rupture may be indicated by the presence of free fluid in the abdomen. A contrast-enhanced abdominal CT scan is the best test to diagnose an AAA and guide treatment options.
Only 10–25% of patients survive rupture due to large pre- and post-operative mortality. Annual mortality from ruptured aneurysms in the United States is about 15,000. Most are due to abdominal aneurysms, with thoracic and thoracoabdominal aneurysms making up 1% to 4% of the total.
Risk factors
- CAD
- Hypertension
- Hypercholesterolemia
- Hyperhomocysteinemia
- Elevated C-reactive protein
- Tobacco use
- Peripheral vascular disease
- Marfan syndrome
- Ehlers-Danlos type IV
- bicuspid aortic valve
Pathophysiology

An aortic aneurysm can occur as a result of trauma, infection, or, most commonly, from an intrinsic abnormality in the elastin and collagen components of the aortic wall.
Prevention
Attention to patient's general blood pressure, smoking and cholesterol risks helps reduce the risk on an individual basis. There have been proposals to introduce ultrasound scans as a screening tool for those most at risk: men over the age of 65.[2][3] The tetracycline antibioticDoxycycline is currently being investigated for use as a potential drug in the prevention of aortic aneurysm due to its metalloproteinase inhibitor and collagen stabilising properties.
A new surgical principle i.e. vascular wall strengthening by endovascular, wide meshes net prosthesis without interference with perfusion of collateral branches,[4] has been hypothesized for preventing aneurysms formation in patients at high risk (Marfan and other genetic disorders) and for very early aortic wall stabilization just at the onset of dilatation in other patients population. Experimental work in pigs in fact showed that in few weeks the endovascular net prosthesis surgically positioned into upper descending aorta is deeply included into the intima thus transferring significant, measurable, wall strengthening while preserving perfusion of some intercostal branches.[5] While the net meshes used in those preliminary experiments where certainly too small to keep long term intercostal branches patency, structural mechanical analysis confirms effectiveness of preventing/arresting aortic wall dilatation of a wider mesh (5x5mm) polipropilene (0,5mm diam) net; on the other end fabric net framework is universally used for preventing dilatation in virtually any elastic structure conducting/confining fluids at high pressure (gardening tube, tires, rubber dinghy etc.). Interestingly enough in this model the endovascular net prosthesis provides aortic wall mechanical support just where the mechanical stress is higher,[6] and thus just where it’s most needed both to prevent further dilatation and to avoid partial (dissection) or total rupture and thus can be viewed as the rational improvement of the very old but still persisting[7] external aortic wall wrapping. Moreover this can be the only method theorethically able to fully prevent paraplegia in extended descending and thoracoabdominal aortic substitution, that clinical experience showed to be not preventable even by the newer endovascular procedures.
Screening
Treatment
Surgery is the definite treatment of aortic aneurysm, while medical therapy is typically supportive.
Surgery
The definitive treatment for an aortic aneurysm may be surgical or endovascular repair. The determination of surgical intervention is complex and determined on a per-case basis. Risk of aneurysm rupture is weighed against procedural risk. The diameter of the aneurysm, its rate of growth, the presence or absence of Marfan Syndrome, Ehlers–Danlos Syndrome or similar connective tissue disorders, and other co-morbidities are all important factors in the overall treatment.
A rapidly expanding aneurysm should under normal circumstances be operated on as soon as feasible, since it has a greater chance of rupture. Slowly expanding aortic aneurysms may be followed by routine diagnostic testing (i.e.: CT scan or ultrasound imaging).
For abdominal aneurysms, the current treatment guidelines for abdominal aortic aneurysms suggest elective surgical repair when the diameter of the aneurysm is greater than 5 cm (2 in). However, recent data on patients aged 60–76 suggest medical management for abdominal aneurysms with a diameter of less than 5.5 cm (2 in).[8]
Open
Open surgery typically involves dissection of the dilated portion of the aorta and insertion of a synthetic (Dacron or Gore-Tex) patch tube. Once the tube is sewn into the proximal and distal portions of the aorta, the aneurysmal sac is closed around the artificial tube. Instead of sewing, the tube ends, made rigid and expandable by nitinol wireframe, can be much more simply, quickly and effectively ("airtight" seal) inserted into the vascular stumps and there permanently fixed by external ligature;[9] different versions of the device were devised for the first (type I) and the second (type II) either distal or proximal aortic anastomosis (video) [1].[10][11] A new version of the expandable anastomotic device (type III) was more recently realized allowing to include the concavity of the aortic arch thus realizing at once distal anastomosis and dacron double strip buttressing with "airtight" dissected layers approximation [12](video). Modified type I expandable devices can be used to quickly connected the supraoertic trunks to the main tube graft when the entire aortic arch needs to be substituted[13](video).[14]
The aorta and possibly also its branching arteries are cross-clamped during open surgery. This can lead to inadequate blood supply to the spinal cord, resulting in neurological deficits. Cerebrospinal fluid drainage (CSFD), when performed in experienced centers, reduce this risk of ischaemic spinal cord injury, as evidenced by randomized trials,[15] by increasing the perfusion pressure to the spinal cord.[16]
Endovascular
In the recent years, the endoluminal treatment of abdominal aortic aneurysms has emerged as a minimally invasive alternative to open surgery repair. The first endoluminal exclusion of an aneurysm took place in Argentina by Dr. Parodi and his colleagues in 1991. The endovascular treatment of aortic aneurysms involves the placement of an endo-vascular stent via a percutaneous technique (usually through the femoral arteries) into the diseased portion of the aorta. This technique has been reported to have a lower mortality rate compared to open surgical repair, and is now being widely used in individuals with co-morbid conditions that make them high risk patients for open surgery. Some centers also report very promising results for the specific method in patients that do not constitute a high surgical risk group.
There have also been many reports concerning the endovascular treatment of ruptured Abdominal Aortic Aneurysms, which are usually treated with an open surgery repair due to the patient's impaired overall condition. Mid-term results have been quite promising.[citation needed] However, due to the time frame of the emerging, the long term benefit of the EVAR procedure against open surgery has not yet been identified.[17]
In spite aneurysms have been treated by endovascular techniques in virtually all aortic segments, better than open aortic repair results were statistically documented only in uncomplicated, elective descending thoracic and infrarenal aorta. Moreover recent USA Nationwide Inpatient Sample data 2006–2007 review of isolated descending thoracic aorta aneurysm cases[18] showed that only 23% (2,563/11,669) of ideal candidate (uncomplicated, elective descending aortic aneurysms) underwent to TEVAR, the remaining 77% (9,106/11,669) still underwent open surgical repair. Although results were better with TEVAR than with OAR it is clear that still the vast majority of thoracic aortic aneurisms is treated by standard open repair.
- Other
The endoluminal exclusion of aortic aneurysms has seen a real revolution in the very recent years. It is now possible to treat thoracic aortic aneurysms, abdominal aortic aneurysms and other aneurysms in most of the body's major arteries (such as the iliac and the femoral arteries) using endovascular stents and avoiding big incisions. Still, in most cases the technique is applied in patients at high risk for surgery as more trials are required to fully accept this method as the gold standard for the treatment of aneurysm.
Medical therapy
Medical therapy of aortic aneurysms involves strict blood pressure control. This does not treat the aortic aneurysm per se, but control of hypertension within tight blood pressure parameters may decrease the rate of expansion of the aneurysm.
See also
References
- ^ Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC (1991). "Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery". Journal of Vascular Surgery : Official Publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 13 (3): 452–8. PMID 1999868.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Routine screening in the management of AAA, UK Department of Health studyReport
- ^ "Abdominal Aortic Aneurysm". Bandolier. 27 (3). May 1996.
- ^ S. Nazari: New approaches for treatment and prevention of thoracic aorta aneurysms. In Front Lines of Thoracic Surgery. S. Nazari Ed, Ch 15 p 263-292, Intech Editor, Feb 2012, ISBN 978-953-307-915-8,DOI: 10.5772/25929
- ^ Nazari S,Luzzana F, Carli F et al (1996c) Aortic wall structural strengthening by intraluminal net prosthesis to arrest aneurysm progression and to prevent dissection and rupture. Experimental assessment of a new therapeutic approach. Eur J Cardiothor Surg, 10:264-272
- ^ Robicsek F & Thubrikar MJ (1994) Hemodynamic considerations regarding the mechanism and prevention of aortic dissection. Ann Thorac Surg 58:1247-1253
- ^ Pepper J, Golesworthy T, Utley M, Chan J, Ganeshalingam S, Lamperth M, Mohiaddin R & Treasure T.(2010) Manufacturing and placing a bespoke support for the Marfan aortic root: description of the method and technical results and status at one year for the first ten patients. Interact Cardiovasc Thorac Surg. 10:360-5. Epub 2009 Dec 11.
- ^ "Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants". Lancet. 352 (9141): 1649–55. 1998. doi:10.1016/S0140-6736(98)10137-X. PMID 9853436.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ S Nazari, F Luzzana, C Banfi, Z Mourad, S Salvi, A Gaspari, F Nazari-Coerezza. Expandable prosthesis for sutureless anastomosis in thoracic aorta prosthetic substitution. Eur J Cardiothor Surg,10:1003-1009, 1996
- ^ Aluffi A, Berti A, Buniva P, Rescigno G, Nazari S (2002). "Improved Device for Sutureless Aortic Anastomosis: Applied in a Case of Cancer". Tex Heart Inst J. 29 (1): 56–9. PMC 101273. PMID 11995854.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Nazari S, Salvi S, Visconti E; et al. (1999). "Descending aorta substitution with expandable ends prosthesis. Case report". J Cardiovasc Surg (Torino). 40 (3): 417–20. PMID 10412932.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nazari S (2010). "Expandable device type III for easy and reliable approximation of dissection layers in sutureless aortic anastomosis. Ex vivo experimental study". Interact Cardiovasc Thorac Surg. 10 (2): 161–4. doi:10.1510/icvts.2009.216291. PMID 19933306.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ S. Nazari, S. Salvi, A. Aluffi, E.Visconti, G.Rescigno, P.Buniva A new prosthesis for aortic arch substitution Ann Thorac Surg, 1997; 64:1339-44
- ^ Nazari, Stefano (2012). Front Lines of Thoracic Surgery. InTech. ISBN 978-953-307-915-8.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15218460, please use {{cite journal}} with
|pmid=15218460instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 14974026, please use {{cite journal}} with
|pmid=14974026instead. - ^ Rutherford RB (2006). "Randomized EVAR trials and advent of level i evidence: a paradigm shift in management of large abdominal aortic aneurysms?". Semin Vasc Surg. 19 (2): 69–74. doi:10.1053/j.semvascsurg.2006.03.001. PMID 16782510.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Gopaldas RR, Huh J, Dao TK; et al. (2010). "Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients". J. Thorac. Cardiovasc. Surg. 140 (5): 1001–10. doi:10.1016/j.jtcvs.2010.08.007. PMID 20951252.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
Bibliography
- Saratzis N, Melas N, Lazaridis J; et al. (2005). "Endovascular AAA repair with the aortomonoiliac EndoFit stent-graft: two years' experience". J Endovasc Ther. 12 (3): 280–7. doi:10.1583/04-1474.1. PMID 15943502.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
External links
- Vascular Medicine — Information for experts and laypersons regarding vascular medicine and related diseases.
- Aortic Aneurysm CT scans MedPix Medical Images
