Acetone peroxide: Difference between revisions
m →top: ce |
m →top: linked to sandbox version of multiple issues template so that the talk page link works |
||
| Line 1: | Line 1: | ||
{{Multiple issues| |
{{Multiple issues/sandbox|talksection = Expert attention needed| |
||
{{expert-subject | Chemistry | date=March 2016 | talk = Expert attention needed }} |
{{expert-subject | Chemistry | date=March 2016 | talk = Expert attention needed }} |
||
{{Refimprove science | date=March 2016 }} |
{{Refimprove science | date=March 2016 }} |
||
Revision as of 10:08, 26 March 2016
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these template messages)
|
 [Cyclic dimer and trimer examples] | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
3,3-Dimethyl-1,2-dioxacyclopropane
(monomer) 3,3,6,6-Tetramethyl-1,2,4,5-tetraoxane (dimer) 3,3,6,6,9,9-Hexamethyl-1,2,4, 5,7,8-hexaoxacyclononane (trimer) 3,3,6,6,9,9,12,12-Octamethyl-1,2,4, 5,7,8,10,11-octaoxacyclododecane (tetramer) | |||
| Other names
Triacetone Triperoxide
Peroxyacetone Mother of Satan | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
| E number | E929 (glazing agents, ...) | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O4 (dimer) C9H18O6 (trimer) C12H24O8 (tetramer) | |||
| Molar mass | 148.157 g/mol (dimer) 222.24 g/mol (trimer) | ||
| Appearance | White crystalline solid | ||
| Melting point | 91 °C (196 °F; 364 K) | ||
| Boiling point | 97 to 160 °C (207 to 320 °F; 370 to 433 K) | ||
| insoluble | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
Explosive | ||
| Explosive data | |||
| Shock sensitivity | High / moderate when wet | ||
| Friction sensitivity | High / moderate when wet | ||
| Detonation velocity | 5300 m/s 17,384 ft/s 3.29 miles per second | ||
| RE factor | 0.83 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Acetone peroxide is an organic peroxide and a primary high explosive. It is produced by the oxidation of acetone to yield a mixture of linear monomer and cyclic dimer, trimer, and tetramer forms. The trimer is known as triacetone triperoxide (TATP) or tri-cyclic acetone peroxide (TCAP). Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach-like odor and can explode if subjected to heat, friction, or shock. As a non-nitrogenous explosive, TATP has historically been more difficult to detect, and it has been implicated as the explosive used in terrorist attacks in Europe in 2016 and earlier.
History
Acetone peroxide (specifically, triacetone triperoxide) was discovered in 1895 by Richard Wolffenstein.[1] He was the first researcher to receive a patent for using the peroxide as an explosive compound.[citation needed]
In 1900 Bayer and Villiger described the first synthesis of the dimer and described use of acids for the synthesis of both peroxides.[2][non-primary source needed] Work on this methodology, and on the various products obtained, was further investigated in the mid-20th century by Milas and Golubović.[3][non-primary source needed]
Chemistry
The chemical name acetone peroxide is most commonly used to refer to the cyclic trimer triacetone triperoxide (TATP; synonyms, tri-cyclic acetone peroxide, TCAP; peroxyacetone), obtained by a reaction between hydrogen peroxide and acetone in an acid-catalyzed nucleophilic addition, although various further monomeric and dimeric forms are possible.[citation needed]
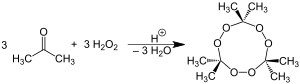
Specifically, two dimers, one cyclic (C6H12O4)[citation needed] and one open chain (C6H14O4), as well as an open chain monomer (C3H8O4),[4] can also be formed; under a particular set of conditions of reagent and acid catalyst concentration, the cyclic trimer is the primary product.[3][non-primary source needed] A tetrameric form has also been described, under different catalytic conditions.[5] Under neutral conditions, the reaction is reported to produce the monomeric organic peroxide.[3][non-primary source needed] Due to significant angle strain of the chemical bonds in the dimer and monomer,[clarification needed] they are even more unstable than the trimer.[citation needed]

Organic peroxides in general are sensitive, dangerous explosives, and all forms of acetone peroxide are sensitive to initiation.[citation needed] TATP decomposes explosively; examination of the explosive decomposition of TATP predicts "formation of acetone and ozone as the main decomposition products and not the intuitively expected oxidation products."[6][non-primary source needed] Very little heat is created by the explosive decomposition of TATP; the foregoing computational analysis suggests that TATP decomposition as an entropic explosion.[6][non-primary source needed] However, this hypothesis has been challenged as not conforming to actual measurements.[7][non-primary source needed] The tetrameric form of acetone peroxide, prepared under neutral conditions using a tin catalyst (with a chelator or general inhibitor of radical chemsitry present), is reported to be more chemically stable, although still a very dangerous primary explosive.[5][non-primary source needed]
Some forms of acetone peroxide are prone to loss by sublimation and evaporation.[citation needed]
Several methods can be used for trace analysis of TATP,[8] including gas chromatography/mass spectrometry (GC/MS)[9][10][11][12][13][non-primary source needed] high performance liquid chromatography/mass spectrometry (HPLC/MS),[14][15][16][17][18][non-primary source needed] and HPLC with post-column derivitization.[19][non-primary source needed]
Industrial uses
Ketone peroxides, including acetone peroxide and methyl ethyl ketone peroxide, find application, alongside other compounds such as benzoyl peroxide, as initiators for polymerization reactions—e.g. silicone or polyester resins, in the making of fiberglass-reinforced composites.[citation needed] For these uses, the peroxides are typically in the form of a dilute solution in an organic solvent; methyl ethyl ketone is more common for this purpose, as it is stable in storage.[citation needed]
Acetone peroxides are common and unwanted by-products of oxidation reactions, such as those used in phenol syntheses.[citation needed]
Acetone peroxide and benzoyl peroxide are used as flour bleaching agents to bleach and "mature" flour.[20]
Due to their explosive nature, their presence in chemical processes creates potential hazardous situations.[citation needed] Numerous methods are used to reduce their appearance as byproducts—for instance, shifting pH to more alkaline, adjusting reaction temperature, or adding inhibitors of their production.[21][non-primary source needed]
Use in improvised explosive devices
TATP and the other explosive forms of acetone peroxide belong to the few high explosives that do not contain nitrogen,[22] and so can pass undetected through scanners designed to detect nitrogenous explosives.[23] Because of its high susceptibility to accidental detonation (and resulting "workplace accidents" in bomb-making shops), TATP has been referred to as the "Mother of Satan."[22] It is used by terrorists for its ability to evade detection aimed at nitrogenous explosives, and due to its low cost and the ease with which its precursors can be obtained.[22] It has been described in popular media as easily prepared from readily available retail ingredients, such as hair bleach and nail polish remover.[24]
As such, TATP has been used in bomb and suicide attacks and in improvised explosive devices, including in Israel,[citation needed] and in the London bombings on 7 July 2005, where four suicide bombers killed 52 people and injured more than 700.[25][26] It was one of the explosives used by the "shoe bomber" Richard Reid[26] and was used by the suicide bombers in the November 2015 Paris attacks[24] and 2016 Brussels bombings.[27]
References
- ^ * Wolffenstein, R (1895). "Über die Einwirkung von Wasserstoffsuperoxyd auf Aceton und Mesityloxyd" [On the effect of hydrogen peroxide on acetone and mesityl oxide]. Berichte der Deutschen Chemischen Gesellschaft (in German). 28 (2): 2265–2269. doi:10.1002/cber.189502802208.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help); Wolfenstein R (1895) Deutsches Reich Patent 84,953; Matyáš, Robert; Pachman, Jiří (2013). Primary Explosives. Berlin: Springer. p. 262. ISBN 978-3-642-28436-6.{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Baeyer, Adolf; Villiger, Victor (1900). "Über die Einwirkung des Caro'schen Reagens auf Ketone". Berichte der deutschen chemischen Gesellschaft. 33 (1): 858–864. doi:10.1002/cber.190003301153.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help);[non-primary source needed] See also Baeyer, Adolf; Villiger, Victor (1900). "Über die Nomenclatur der Superoxyde und die Superoxyde der Aldehyde". Berichte der deutschen chemischen Gesellschaft. 33 (2): 2479–2487. doi:10.1002/cber.190003302185.{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help); Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)[non-primary source needed] - ^ a b c Milas NA, Golubović A (1959). "Studies in Organic Peroxides. XXVI. Organic Peroxides Derived from Acetone and Hydrogen Peroxide". Journal of the American Chemical Society. 81 (24): 6461–6462. doi:10.1021/ja01533a033.[non-primary source needed]
- ^ This is not the DMDO monomer referred to in the Chembox, but rather the open chain, dihydro monomer described by Milas & Goluboviç, op. cit.
- ^ a b Jiang H, Chu G, Gong H, Qiao Q (1999). "Tin Chloride Catalysed Oxidation of Acetone with Hydrogen Peroxide to Tetrameric Acetone Peroxide". Journal of Chemical Research. 28 (4): 288–289. doi:10.1039/a809955c.
- ^ a b Dubnikova F, Kosloff R, Almog J, Zeiri Y, Boese R, Itzhaky H, Alt A, Keinan E (February 2005). "Decomposition of triacetone triperoxide is an entropic explosion" (PDF). Journal of the American Chemical Society. 127 (4): 1146–59. doi:10.1021/ja0464903. PMID 15669854.
- ^ Sinditskii VP, Koltsov VI, Egorshev, VY, Patrikeev DI, Dorofeeva OV (2014). "Thermochemistry of cyclic acetone peroxides". Thermochimica Acta. 585: 10–15. doi:10.1016/j.tca.2014.03.046.
- ^ Schulte-Ladbeck R, Vogel M, Karst U (October 2006). "Recent methods for the determination of peroxide-based explosives". Analytical and Bioanalytical Chemistry. 386 (3): 559–65. doi:10.1007/s00216-006-0579-y. PMID 16862379.
- ^ Muller D, Levy A, Shelef R, Abramovich-Bar S, Sonenfeld D, Tamiri T (September 2004). "Improved method for the detection of TATP after explosion". Journal of Forensic Sciences. 49 (5): 935–8. PMID 15461093.
- ^ Stambouli A, El Bouri A, Bouayoun T, Bellimam MA (December 2004). "Headspace-GC/MS detection of TATP traces in post-explosion debris". Forensic Science International. 146 Suppl: S191-4. doi:10.1016/j.forsciint.2004.09.060. PMID 15639574.
- ^ Oxley, Jimmie C.; Smith, James L.; Shinde, Kajal; Moran, Jesse (2005). "Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Technique". Propellants, Explosives, Pyrotechnics. 30 (2): 127. doi:10.1002/prep.200400094.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sigman ME, Clark CD, Fidler R, Geiger CL, Clausen CA (2006). "Analysis of triacetone triperoxide by gas chromatography/mass spectrometry and gas chromatography/tandem mass spectrometry by electron and chemical ionization". Rapid Communications in Mass Spectrometry. 20 (19): 2851–7. doi:10.1002/rcm.2678. PMID 16941533.
- ^ Romolo FS, Cassioli L, Grossi S, Cinelli G, Russo MV (January 2013). "Surface-sampling and analysis of TATP by swabbing and gas chromatography/mass spectrometry". Forensic Science International. 224 (1–3): 96–100. doi:10.1016/j.forsciint.2012.11.005. PMID 23219697.
- ^ Widmer L, Watson S, Schlatter K, Crowson A (December 2002). "Development of an LC/MS method for the trace analysis of triacetone triperoxide (TATP)". The Analyst. 127 (12): 1627–32. doi:10.1039/B208350G. PMID 12537371.
- ^ Xu X, van de Craats AM, Kok EM, de Bruyn PC (November 2004). "Trace analysis of peroxide explosives by high performance liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry (HPLC-APCI-MS/MS) for forensic applications". Journal of Forensic Sciences. 49 (6): 1230–6. PMID 15568694.
- ^ Cotte-Rodríguez I, Hernandez-Soto H, Chen H, Cooks RG (March 2008). "In situ trace detection of peroxide explosives by desorption electrospray ionization and desorption atmospheric pressure chemical ionization". Analytical Chemistry. 80 (5): 1512–9. doi:10.1021/ac7020085. PMID 18247583.
- ^ Sigman ME, Clark CD, Caiano T, Mullen R (2008). "Analysis of triacetone triperoxide (TATP) and TATP synthetic intermediates by electrospray ionization mass spectrometry". Rapid Communications in Mass Spectrometry. 22 (2): 84–90. doi:10.1002/rcm.3335. PMID 18058960.
- ^ Sigman ME, Clark CD, Painter K, Milton C, Simatos E, Frisch JL, McCormick M, Bitter JL (February 2009). "Analysis of oligomeric peroxides in synthetic triacetone triperoxide samples by tandem mass spectrometry". Rapid Communications in Mass Spectrometry. 23 (3): 349–56. doi:10.1002/rcm.3879. PMID 19125413.
- ^ Schulte-Ladbeck R, Kolla P, Karst U (February 2003). "Trace analysis of peroxide-based explosives". Analytical Chemistry. 75 (4): 731–5. doi:10.1021/ac020392n. PMID 12622359.
- ^ Ferrari, C. G.; Higashiuchi, K.; Podliska, J. A. (1963). "Flour Maturing and Bleaching with Acyclic Acetone Peroxides" (PDF). Cereal Chemistry. 40: 89–100.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Costantini, Michel (1991-03-26) Destruction of acetone peroxide. United States Patent 5003109. Freepatentsonline.com. Retrieved on 2013-02-03.[non-primary source needed]
- ^ a b c "TATP: Countering the Mother of Satan". The Future of Things. 6 November 2006. Retrieved 24 September 2009.
The tremendous devastative force of TATP, together with the relative ease of making it, as well as the difficulty in detecting it, made TATP one of the weapons of choice for terrorists
{{cite web}}: Cite uses deprecated parameter|authors=(help) - ^ "Feds are all wet on airport security". Star-Ledger (Newark, New Jersey). 24 August 2006. Retrieved 11 September 2009.
At the moment, Watts said, the screening devices are set to detect nitrogen-based explosives, a category that doesn't include TATP
- ^ a b Callimachi, Rukmini; Rubin, Alissa J.; Fourquet, Laure (19 March 2016). "A View of ISIS's Evolution in New Details of Paris Attacks". The New York Times.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Naughton, Philippe (15 July 2005). "TATP is suicide bombers' weapon of choice". The Times (UK). Archived from the original on 10 February 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help); Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Vince, Gaia (15 July 2005). "Explosives linked to London bombings identified". New Scientist.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ ""La mère de Satan" ou TATP, l'explosif préféré de l'EI" ["Mother of Satan " or TATP , the preferred explosive of IE]. LeVif.be Express (in French).


