Pulsed electromagnetic field therapy: Difference between revisions
Magenta1984 (talk | contribs) |
Magenta1984 (talk | contribs) |
||
| Line 12: | Line 12: | ||
'''PEMF as Wellness Devices''' |
'''PEMF as Wellness Devices''' |
||
The original PEMF devices consisted of a [[Helmholtz coil]] and your body is placed inside the magnetic field to get a treatment. Today, the majority of PEMF wellness devices resemble a typical yoga mat in dimensions but are slightly thicker to house several flat spiral Tesla coils to produce an even electromagnetic field. A [[Signal generator|frequency generator]] is then used to energize the coils to create a "pulsed" electromagnetic field. Today, there is a wide variety of professional and consumer PEMF devices that are sold and marketed on the internet as FDA registered [http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM429674.pdf wellness devices]. The majority are manufactured in Germany, Austria and Switzerland and are imported |
The original PEMF devices consisted of a [[Helmholtz coil]] and your body is placed inside the magnetic field to get a treatment. Today, the majority of PEMF wellness devices resemble a typical yoga mat in dimensions but are slightly thicker to house several flat spiral Tesla coils to produce an even electromagnetic field. A [[Signal generator|frequency generator]] is then used to energize the coils to create a "pulsed" electromagnetic field. Today, there is a wide variety of professional and consumer PEMF devices that are sold and marketed on the internet as FDA registered [http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM429674.pdf wellness devices]. The majority are manufactured in Germany, Austria and Switzerland and are imported as electric massage or full body electric yoga mats. They are either placed on a massage table for clinical use or directly on the floor to practice simple yoga postures. The companies that sell and manufacture them make no medical claims as to their effectiveness in treating disease mainly to bypass FDA clearance medical device regulations and approvals.<ref>{{cite web | url=http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4224b1-10-TabE-FDA-Executive-Summary.pdf | title=FDA Executive Summary - Orthopaedic and Rehabilitation Devices Panel | publisher=Fda.gov | accessdate=13 May 2014}}</ref> |
||
== Use == |
== Use == |
||
Revision as of 17:28, 20 July 2016
This article needs more reliable medical references for verification or relies too heavily on primary sources. (January 2016) |  |

Pulsed electromagnetic field therapy (PEMFT), also called pulsed magnetic therapy, pulse magnetotherapy, or PEMF, is a reparative technique most commonly used in the field of orthopedics for the treatment of non-union fractures, failed fusions, congenital pseudarthrosis and depression.[1] In the case of bone healing, PEMF uses directed pulsed magnetic fields through injured tissue to stimulate cellular repair. The FDA has cleared several such stimulation devices.[2][3]
History
Electromagnetic field therapy has been in use since the invention of electricity. It was widely adopted in East and Western Europe but its use was restricted to animals in North America until recently.[4] Veterinarians became the first health professionals to use PEMF therapy, usually to heal broken legs in racehorses. Professional sports doctors then decided to experiment with veterinarian devices off label on professional athletes which ultimately led to legally FDA licensed devices in 1979 to stimulate bone growth.[5]
In 2004, pulsed electromagnetic field system was approved by FDA as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.[5] On 10/13/2015 the FDA reclassified PEMF devices from their existing Class 3 category to a Class 2 status. PEMF devices that have been FDA cleared to make health claims require a doctors prescription for use.
PEMF as Wellness Devices
The original PEMF devices consisted of a Helmholtz coil and your body is placed inside the magnetic field to get a treatment. Today, the majority of PEMF wellness devices resemble a typical yoga mat in dimensions but are slightly thicker to house several flat spiral Tesla coils to produce an even electromagnetic field. A frequency generator is then used to energize the coils to create a "pulsed" electromagnetic field. Today, there is a wide variety of professional and consumer PEMF devices that are sold and marketed on the internet as FDA registered wellness devices. The majority are manufactured in Germany, Austria and Switzerland and are imported as electric massage or full body electric yoga mats. They are either placed on a massage table for clinical use or directly on the floor to practice simple yoga postures. The companies that sell and manufacture them make no medical claims as to their effectiveness in treating disease mainly to bypass FDA clearance medical device regulations and approvals.[6]
Use
Delayed- and non-union fractures
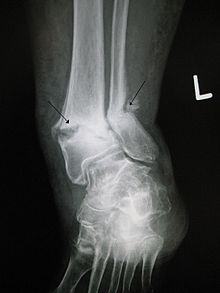
In 1974 it was demonstrated that a pulsed magnetic field applied across the site of a bone fracture can accelerate the healing process (Bassett et al., 1974). The mechanism of osteogenesis is not clear; however, the use of PEMF therapy as an adjuvant therapy for delayed- and non-union fractures was supported by empirical evidence collected through clinical studies.[7][8] While PEMF therapy may offer some benefit in the treatment of fractures, the evidence is inconclusive and is insufficient to inform current clinical practice.[9]
Although electricity’s potential to aid bone healing was reported as early as 1841, it was not until the mid-1950s that scientists seriously studied the subject. Fukada’s and Yasuda’s discovery of the electric potential of bone provided evidence of electricity’s effect in promoting osteogenesis (bone growth), particularly in long bone non-unions.[10] During the 1970s, Bassett and his team introduced a new approach for the treatment of delayed fractures, a technique that employed a very specific biphasic low frequency signal [11][12][13][14] to be applied for non-union/delayed fractures. The use of electrical stimulation in the lumbosacral region was first attempted by Alan Dwyer of Australia. In 1974, he reported successful initiation of graft incorporation in 11 of 12 fusion patients. Since that time, electrical stimulation has been shown to significantly increase the probability of bony arthrodesis in spinal fusions.[15][16]
Depression
Transcranial magnetic stimulation is FDA approved since 2011 for use on patients that failed to respond to antidepressants.[17] Weak magnetic stimulation of the brain is often called transcranial pulsed electromagnetic field (tPEMF) therapy.[18] Early hints of efficacy of tPEMF as antidepressant came from observations that bipolar patients improved their mood after a magnetic resonance spectrogram, an approach which has been labeled LFMS.[19] Studies in rodents have shown behavioural effects of tPEMF that are consistent with antidepressant effects.[20][21][22] In humans, the treatment is usually given once or twice daily for two or more weeks.[18]
References
- ^ Bassett CA, Schink-Ascani M, Lewis SM (September 1989). "Effects of Pulsed Electromagnetic Fields on Steinberg Ratings of Femoral Head Osteonecrosis". Columbia Presbyterian Medical Center.
- ^ Markov, Marko S (2007). "Expanding Use of Pulsed Electromagnetic Field Therapies". Electromagnetic Biology & Medicine. 26 (3): 257–274. doi:10.1080/15368370701580806. PMID 17886012.
- ^ Mooney, V (1990). "A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions". Spine. 15 (7): 708–712. doi:10.1097/00007632-199007000-00016. PMID 2218718.
- ^ Ross Roeser; Michael Valente (2007). AUDIOLOGY, 3-Volume Set: Diagnosis, Treatment and Practice Management. 342: Thieme. p. 2000. ISBN 1604066970.
{{cite book}}: CS1 maint: location (link) - ^ a b "Electrical stimulation of the spine as an adjunct to spinal fusion procedures". Blue Cross & Blue Shield of Mississippi.
Pulsed electromagnetic field systems with FDA PMA include the EBI Bone Healing System from Electrobiology, Inc., which was first approved in 1979 and indicated for nonunions, failed fusions, and congenital pseudarthroses; and the Cervical-Stim from Orthofix, which was approved in 2004 as an adjunct to cervical fusion surgery in patients at high risk for non-fusion.
- ^ "FDA Executive Summary - Orthopaedic and Rehabilitation Devices Panel" (PDF). Fda.gov. Retrieved 13 May 2014.
- ^ Boopalan, PRJVC; Chittaranjan, Samuel B; Balamurugan, Ramadass; Nandakumar, NS; Sabareeswaran, A; Mohanty, Mira (August 2009). "Pulsed electromagnetic field (PEMF) treatment for fracture healing". Current Orthopaedic Practice. 20 (4): 423–428. doi:10.1097/BCO.0b013e318198e8b2.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ "Is there still a role for pulsed electromagnetic field in the treatment of delayed unions and nonunions". The Internet Journal of Orthopedic Surgery. 10 (1). 2008.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Griffin, XL; Costa, ML; Parsons, N; Smith, N (13 April 2011). "Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults". The Cochrane database of systematic reviews (4): CD008471. doi:10.1002/14651858.CD008471.pub2. PMID 21491410.
- ^ Inoue, S.; Ohashi, T.; Yasuda, I.; Fukada, E. (1977). "Electret induced callus formation in the rat". Clinical Orthopaedics and Related Research. 124 (124): 57–58. doi:10.1097/00003086-197705000-00008. PMID 598094.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - ^ Bassett CA, Pawluk RJ, Pilla AA; Pawluk; Pilla (1974). "Acceleration of fracture repair by electromagnetic fields. A surgically noninvasive method". Ann N Y Acad Sci. 238: 242–62. Bibcode:1974NYASA.238..242B. doi:10.1111/j.1749-6632.1974.tb26794.x. PMID 4548330.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bassett CA, Pawluk RJ, Pilla AA; Pawluk; Pilla (1974). "Augmentation of Bone Repair by Inductively Coupled Electromagnetic Fields". Science. 184 (4136): 575–7. Bibcode:1974Sci...184..575B. doi:10.1126/science.184.4136.575. PMID 4821958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bassett CA, Pilla AA, Pawluk RJ; Pilla; Pawluk (1977). "A non-operative salvage of surgically-resistant pseudarthroses and non-unions by pulsing electromagnetic fields. A preliminary report". Clin Orthop. 124 (124): 128–43. doi:10.1097/00003086-197705000-00017. PMID 598067.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bassett CA, Mitchell SN, Norton L, Pilla A; Mitchell; Norton; Pilla (1978). "Repair of non-unions by pulsing electromagnetic fields". Acta Orthop Belg. 44 (5): 706–24. PMID 380258.
{{cite journal}}: CS1 maint: multiple names: authors list (link)[unreliable medical source?] - ^ Mackenzie, Donald, Francis D Veninga; Veninga (2004). "Reversal of delayed union of anterior cervical fusion treated with pulsed electromagnetic field stimulation: case report". Southern Medical Journal. 97 (5): 519–524. doi:10.1097/00007611-200405000-00021. PMID 15180031.
{{cite journal}}: CS1 maint: multiple names: authors list (link)[unreliable medical source?] - ^ Bose, B (2001). "Outcomes after posterolateral lumbar fusion with instrumentation in patients treated with adjunctive pulsed electromagnetic field stimulation". Advances in Therapy. 18 (1): 12–20. doi:10.1007/BF02850247. PMID 11512529.[unreliable medical source?]
- ^ NeuroStar TMS Therapy system
- ^ a b Martiny K, Lunde M, Bech P.; Lunde; Bech (2010). "Transcranial low voltage pulsed electromagnetic fields in patients with treatment-resistant depression". Society of Biological Psychiatry. 68 (2): 163–9. doi:10.1016/j.biopsych.2010.02.017. PMID 20385376.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rohan M, Parow A, Stoll AL, Demopulos C, Friedman S, Dager S, Hennen J, Cohen BM, Renshaw PF (2004). "Low-field magnetic stimulation in bipolar depression using an MRI-based stimulator". Am J Psychiatry. 161 (1): 93–8. doi:10.1176/appi.ajp.161.1.93. PMID 14702256.
- ^ Aksoz E, Aksoz T, Bilge SS, Ilkaya F, Celik S, Diren HB (2008). "Antidepressant-like effects of echo-planar magnetic resonance imaging in mice determined using the forced swimming test". Brain Res. 1236: 194–9. doi:10.1016/j.brainres.2008.08.011. PMID 18755160.
- ^ Rokni-Yazdi H, Sotoudeh H, Akhondzadeh S, Sotoudeh E, Asadi H, Shakiba M (2007). "Prog Neuropsychopharmacol". Biol Psychiatry. 31 (2): 503–9. doi:10.1016/j.pnpbp.2006.11.021. PMID 17218047.
- ^ Carlezon WA Jr; Rohan ML; Mague SD; Meloni EG; Parsegian A; Cayetano K; Tomasiewicz HC; Rouse ED; Cohen BM; Renshaw PF. (2005). "Antidepressant-like effects of cranial stimulation within a low-energy magnetic field in rats". Biol Psychiatry. 57 (6): 571–6. doi:10.1016/j.biopsych.2004.12.011. PMID 15780843.
Further reading
- Vavken, Patrick; Arrich, Ferdi; Schuhfried, Othmar; Dorotka, Ronald (2009-05-01). "Effectiveness of pulsed electromagnetic field therapy in the management of osteoarthritis of the knee: a meta-analysis of randomized controlled trials". Journal of Rehabilitation Medicine. 41 (6): 406–411. doi:10.2340/16501977-0374. ISSN 1651-2081. PMID 19479151.
