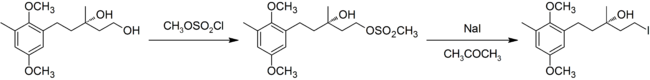Finkelstein reaction: Difference between revisions
wlink |
→Method: added informations Tags: Mobile edit Mobile app edit Android app edit |
||
| Line 13: | Line 13: | ||
==Method== |
==Method== |
||
The classic Finkelstein reaction entails the conversion of an [[alkyl chloride]] or an [[alkyl bromide]] to an [[alkyl iodide]] by treatment with a solution of [[sodium iodide]] in [[acetone]]. Sodium iodide is soluble in acetone while [[sodium chloride]] and [[sodium bromide]] are not.<ref>{{cite journal | last = Ervithayasuporn | first = V. | year = 2013 | title = One-pot synthesis of halogen exchanged silsesquioxanes: octakis(3-bromopropyl)octasilsesquioxane and octakis(3-iodopropyl)octasilsesquioxane | journal = [[Dalton Trans.]] | volume = 42 | issue = 37 | pages = 13747–13753 | doi = 10.1039/C3DT51373D }}</ref> The reaction is driven toward products by [[Law of mass action|mass action]] due to the precipitation of the poorly soluble NaCl or NaBr. An example involves the conversion of the ethyl ester of 5-bromo[[valeric acid]] to the iodide:<ref>{{cite journal|title=Copper-catalyzed Conjugate Addition of Functionalized Organozinc Reagents to α,β-Unsaturated Ketones: Ethyl 5-(3-oxocyclohexyl)pentanoate|authors=B. H. Lipshutz, M. R. Wood, and R. Tirado |
The classic Finkelstein reaction entails the conversion of an [[alkyl chloride]] or an [[alkyl bromide]] to an [[alkyl iodide]] by treatment with a solution of [[sodium iodide]] in [[acetone]]. Sodium iodide is soluble in acetone while [[sodium chloride]] and [[sodium bromide]] are not.<ref>{{cite journal | last = Ervithayasuporn | first = V. | year = 2013 | title = One-pot synthesis of halogen exchanged silsesquioxanes: octakis(3-bromopropyl)octasilsesquioxane and octakis(3-iodopropyl)octasilsesquioxane | journal = [[Dalton Trans.]] | volume = 42 | issue = 37 | pages = 13747–13753 | doi = 10.1039/C3DT51373D }}</ref> |
||
In this reaction, Iodine replaces Br and Cl instead of being a better leaving group. This may creat ambiguity but one needs to understand that reaction wpuld have gone in both directions. However, the reaction goes towards product because of insolubility of NaBr and NaCl in acetone. |
|||
The reaction is driven toward products by [[Law of mass action|mass action]] due to the precipitation of the poorly soluble NaCl or NaBr. An example involves the conversion of the ethyl ester of 5-bromo[[valeric acid]] to the iodide:<ref>{{cite journal|title=Copper-catalyzed Conjugate Addition of Functionalized Organozinc Reagents to α,β-Unsaturated Ketones: Ethyl 5-(3-oxocyclohexyl)pentanoate|authors=B. H. Lipshutz, M. R. Wood, and R. Tirado |
|||
|journal=Org. Synth.|year=1999|volume=76|page=252 |
|journal=Org. Synth.|year=1999|volume=76|page=252 |
||
|doi=10.15227/orgsyn.076.0252}}</ref> |
|doi=10.15227/orgsyn.076.0252}}</ref> |
||
Revision as of 07:50, 13 October 2019
| Finkelstein reaction | |
|---|---|
| Named after | Hans Finkelstein |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | finkelstein-reaction |
| RSC ontology ID | RXNO:0000155 |
The Finkelstein reaction (often referred to as a halex reaction or halogen exchange) named after the German chemist Hans Finkelstein,[1] is an SN2 reaction (Substitution Nucleophilic Bimolecular reaction) that involves the exchange of one halogen atom for another. It is an equilibrium reaction, but the reaction can be driven to completion by exploiting the differential solubility of halide salts, or by using a large excess of the halide salt.[2]
- R–X + X′− ⇌ R–X′ + X−
Method
The classic Finkelstein reaction entails the conversion of an alkyl chloride or an alkyl bromide to an alkyl iodide by treatment with a solution of sodium iodide in acetone. Sodium iodide is soluble in acetone while sodium chloride and sodium bromide are not.[3] In this reaction, Iodine replaces Br and Cl instead of being a better leaving group. This may creat ambiguity but one needs to understand that reaction wpuld have gone in both directions. However, the reaction goes towards product because of insolubility of NaBr and NaCl in acetone. The reaction is driven toward products by mass action due to the precipitation of the poorly soluble NaCl or NaBr. An example involves the conversion of the ethyl ester of 5-bromovaleric acid to the iodide:[4]
- EtO2C(CH2)4Br + NaI → EtO2C(CH2)4I + NaBr
Use for analysis
Alkyl halides differ greatly in the ease with which they undergo the Finkelstein reaction. The reaction works well for primary (except for neopentyl) halides, and exceptionally well for allyl, benzyl, and α-carbonyl halides. Secondary halides are far less reactive. Vinyl, aryl and tertiary alkyl halides are unreactive; as a result, the reaction of NaI in acetone can be used as a qualitative test to determine which of the aforementioned classes an unknown alkyl halide belongs to, with the exception of alkyl iodides, as they yield the same product upon substitution. Below some relative rates of reaction (NaI in acetone at 60 °C):[5][6]
| Me–Cl | Bu–Cl | i-Pr–Cl | t-BuCH2–Cl | CH2=CH–CH2–Cl | PhCH2–Cl | EtOC(O)CH2–Cl | MeC(O)CH2–Cl |
|---|---|---|---|---|---|---|---|
| 179 | 1 | 0.0146 | 0.00003 | 64 | 179 | 1600 | 33000 |
In modern usage the definition of the reaction has been expanded to include the conversion of alcohols to alkyl halides by first converting the alcohol to a sulfonate ester (tosylates or mesylates are usually used), and then performing the substitution. The example below is from a synthesis of chrysochlamic acid.[7]
Aromatic Finkelstein reaction
The aromatic chlorides and bromides are not easily substituted by iodide, though they may occur when appropriately catalyzed. The so-called "aromatic Finkelstein reaction" is catalyzed by copper(I) iodide in combination with diamine ligands.[8] Nickel bromide and tri-n-butylphosphine have been found to be suitable catalysts as well.[9]
References
- ^ Finkelstein, Hans (1910). "Darstellung organischer Jodide aus den entsprechenden Bromiden und Chloriden". Ber. Dtsch. Chem. Ges. (in German). 43 (2): 1528–1532. doi:10.1002/cber.19100430257.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Ervithayasuporn, V. (2013). "One-pot synthesis of halogen exchanged silsesquioxanes: octakis(3-bromopropyl)octasilsesquioxane and octakis(3-iodopropyl)octasilsesquioxane". Dalton Trans. 42 (37): 13747–13753. doi:10.1039/C3DT51373D.
- ^ "Copper-catalyzed Conjugate Addition of Functionalized Organozinc Reagents to α,β-Unsaturated Ketones: Ethyl 5-(3-oxocyclohexyl)pentanoate". Org. Synth. 76: 252. 1999. doi:10.15227/orgsyn.076.0252.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Streitwieser, A. (1956). "Solvolytic Displacement Reactions at Saturated Carbon Atoms". Chem. Rev. 56 (4): 571–752. doi:10.1021/cr50010a001.
- ^ Bordwell, F. G.; Brannen, W. T. (1964). "The Effect of the Carbonyl and Related Groups on the Reactivity of Halides in SN2 Reactions". J. Am. Chem. Soc. 86 (21): 4645–4650. doi:10.1021/ja01075a025.
- ^ Maloney, D. J.; Hecht, S. M. (2005). "A Stereocontrolled Synthesis of δ-trans-Tocotrienoloic Acid". Org. Lett. 7 (19): 4297–300. doi:10.1021/ol051849t. PMID 16146411.
- ^ Klapars, A.; Buchwald, S. L. (2002). "Copper-Catalyzed Halogen Exchange in Aryl Halides: An Aromatic Finkelstein Reaction". J. Am. Chem. Soc. 124 (50): 14844–14845. doi:10.1021/ja028865v. PMID 12475315.
- ^ Cant, Alastair A.; Bhalla, Rajiv; Pimlott, Sally L.; Sutherland, Andrew (2012). "Nickel-catalysed aromatic Finkelstein reaction of aryl and heteroaryl bromides". Chem. Commun. 48 (33): 3993–5. doi:10.1039/c2cc30956d. PMID 22422214.